Abstract
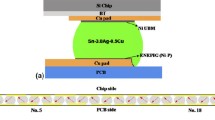
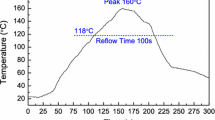
Micro-bumps are now being developed with diameters smaller than 10 μm. At these dimensions, only very small amounts of solder are used to form the interconnections. Surface oxidation of such small micro-bumps is a critical issue. The key question is whether the oxide film on the solder bumps acts as a barrier to formation of solder joints. In this work, the mechanical stability of the oxide layer on solder bumps was investigated. Solder bumps with 35-μm radii were heated for different times. Auger electron spectroscopy was used to determine the thickness of the oxide layer on the solder bumps. Solder bumps with known oxide layer thicknesses were then heated in a low-oxygen environment (<50 ppm) until they melted. The mechanical stability of the oxide layer was observed by use of a high-speed camera. Results showed that a 14-nm-thick oxide layer on a solder bump of radius 35 μm was able to withstand the molten solder without cracking, leading to a non-wetting solder joint. A thermal stress model of the surface oxide layer revealed that the stress varied substantially with bump size and temperature, and increased almost linearly with temperature. Upon melting, the thermal stress on the oxide increased abruptly, because of the higher thermal expansion of molten solder compared with its solid state. On the basis of the experimental results and the thermal stress model of the oxide film, the maximum oxide thickness that can be tolerated to form a solder joint was determined, e.g. 14 nm oxide can support liquid solder, and thus lead to a non-wetting condition. This work provided a new method of determination of the maximum stress of oxide film for solder joint formation.
Similar content being viewed by others
References
R. Chanchani, Materials for Advanced Packaging, ed. D. Lu and C.P. Wong (Boston: Springer, 2009), pp. 4–27.
K.N. Tu, Microelectron. Reliab. 51, 517 (2011).
C.K. Chung, Y.J. Chen, C.C. Li, and C.R. Kao, Thin Solid Films 520, 5346 (2012).
X. Luo, W. Du, X. Lu, T. Yamaguchi, J. Gavin, L. Ye, and J. Liu, IEEE International Symposium on Advanced Packaging Materials, Xiamen, China October 25–28 (2011), pp. 73–79.
S. Zhang, Y. Zhang, and H. Wang, J. Alloys Compd. 487, 682 (2009).
S. Cho, J. Yu, S.K. Kang, and D.-Y. Shih, JOM 57, 50 (2005).
F.Y. Hung, H.-M. Lin, P.-S. Chen, T.-S. Lui, and L.-H. Chen, J. Alloys Compd. 415, 85 (2006).
J. Gorlich, C.C. Oberdorfer, D. Baither, G. Schmitz, C. Reinke, and U. Wilke, J. Alloys Compd. 490, 336 (2010).
M. Abtew and G. Selvaduray, Mat. Sci. Eng. R 27, 95 (2000).
C.M.L. Wu, D.Q. Yu, C.M.T. Law, and L. Wang, Mater. Sci. Eng., R 44, 1 (2004).
T. Laurila, V. Vuorinen, and M. Paulasto-Krockel, Mater. Sci. Eng., R 68, 1 (2010).
J. Zhou, D. Huang, Y.-L. Fang, and F. Xue, J. Alloys Compd. 480, 903 (2009).
M.A. Dudek and N. Chawla, J. Electron. Mater. 38, 210 (2009).
C.K. Chung, T.C. Huang, R. Shia, T.L. Yang, and C.R. Kao, J. Alloys Compd. 539, 57 (2012).
C.-H. Hsueh and A.G. Evans, J. Appl. Phys. 54, 6672 (1983).
S.M. Hu, J. Appl. Phys. 64, 323 (1988).
C.S. Rafferty and R.W. Dutton, Appl. Phys. Lett. 54, 1815 (1989).
C.-H. Hsueh and S. Lee, J. Appl. Phys. 91, 2760 (2002).
K.-S. Kim, K. Suganuma, J.-M. Kim, and C.-W. Hwang, JOM 56, 39 (2004).
R. DeHoff, Thermodynamic in Materials Science, 2nd edn. (New York: CRC Press Taylor & Francis, 2006), Chap. 4 and 12.
P.G. Harrison, Chemistry of Tin, ed. P.J. Smith (New York: Springer, 1998), pp. 1–9.
K.H. Schramm, Z. Metallk. 53, 316 (1962).
P.L. Clavette, A Study of Tin Wisker Growth in Electronics (MSc thesis, Mech. Eng. Rensselaer Polytechnic Institute, August 2009).
B. Saatci, S. Cimen, H. Pamuk, and M. Gündüz, J. Phys.: Condens. Matter 19, 326219 (2007).
S. Sharafat and N. Ghoniem, Summary of Thermo-Physical Properties of Sn and its Compounds (Uni. of California L.A., 2000), pp. 8–23.
O. Madelung, U. Rössler, and M. Schulz, Tin Dioxide (SnO 2 ) Crystal Structure, Lattice Parameters, Thermal Expansion (The Landolt-Börnstein database-Group III Condensed Matter, Vol III 17E, 17F-41C) Chap. T1.
S. Barth, C. Harnagea, S. Mathur, and F. Rosei, Nanotechnology 20, 115705 (2009).
C.K. Chung, Y.J. Chen, T.L. Yang, and C.R. Kao, J. Electron. Mater. 42, 1254 (2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Key Chung, C., Zhu, Z.X. & Kao, C.R. Thermal Stress of Surface Oxide Layer on Micro Solder Bumps During Reflow. J. Electron. Mater. 44, 744–750 (2015). https://doi.org/10.1007/s11664-014-3528-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-014-3528-0