Abstract
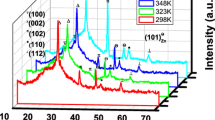
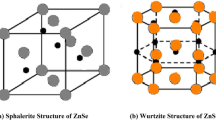
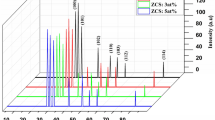
Nanocrystalline ZnSe films were prepared by the chemical bath deposition technique by varying the pH of the reaction bath from 9.2 to 10.6, and the physical properties of the annealed films were investigated. The x-ray diffraction profiles of the samples showed hexagonal structure of ZnSe nanocrystallites except for films deposited at pH 10.6. Strain was compressive, but became tensile with increase in pH. Topographical studies by atomic force microscopy showed a smooth surface of the samples deposited at higher pH and therefore low surface roughness. Optical studies revealed homogeneous grain shape and size. The thickness, grain size, and bandgap of the samples showed consistent results. The photoluminescence emission peaks occurred at the same wavelength for different pH values. The physical properties of the annealed samples seem to make them suitable for application as buffer layers in thin-film solar cells.
Similar content being viewed by others
References
A.R. Balu, V.S. Nagarethinam, M.G. Syed Basheer Ahmed, A. Thayumanavan, and K.R. Murali, Mater. Sci. Eng. B 171, 93 (2010).
C.W. Huang, H.M. Weng, Y.U. Jiang, and H.Y. Heng, Vacuum 83, 313 (2009).
C. Mehta, G.S.S. Saini, J.M. Abbas, and S.K. Tripathi, Appl. Surf. Sci. 256, 608 (2009).
L. Chen, D. Zhang, G. Zhai, and J. Zhang, Mater. Chem. Phys. 120, 456 (2010).
W. Wang, S.Y. Han, S.J. Sung, D.H. Kim, and C.H. Chang, Phys. Chem. Chem. Phys. 14, 11154 (2012).
S.W. Shin, S.R. Kang, J.H. Yun, A.V. Moholkar, J.H. Moon, J.Y. Lee, and J.H. Kim, Sol. Energy Mater. Sol. Cells 95, 856 (2011).
G.I. Rusu, V. Ciupina, M.E. Popa, G. Prodan, G.G. Rusu, and C. Baban, J. Non-Cryst. Solids 352, 1525 (2006).
M.J. Bevan, H.D. Shih, J.A. Dodge, A.J. Syllaios, and F. Weirauch, J. Electron. Mater. 27, 769 (1998).
T. Zhang, N. Xu, Y. Shen, W. Hu, J. Wu, J. Sun, and Z. Ying, J. Electron. Mater. 36, 75 (2007).
M. Oztas and M. Bedir, Mater. Lett. 61, 343 (2007).
J. Hai-qing, C.H. Jun, and Y. Xi, Trans. Nonferr. Met. Soc. China 16, 266 (2006).
R.B. Kale and C.D. Lokhande, Mater. Res. Bull. 39, 1829 (2004).
P.P. Hankare, P.A. Chate, P.A. Chavan, and D.J. Sathe, J. Alloys Compd. 461, 623 (2008).
R.B. Kale and C.D. Lokhande, Appl. Surf. Sci. 252, 929 (2005).
R.B. Kale, C.D. Lokhande, R.S. Mane, and S.H. Han, Appl. Surf. Sci. 252, 5768 (2006).
C.D. Lokhande, P.S. Patilet, A. Ennaoui, and H. Tributsch, Appl. Surf. Sci. 123, 294 (1998).
L. Chen, D. Zhang, G. Zhai, and J. Zhang, Mater. Chem. Phys. 120, 56 (2010).
J. Mazher, A.K. Srivastav, R.V. Nandedkar, and R.K. Pandey, Nanotechnology 15, 572 (2004).
A. Karipera, E. Guneria, F. Godeb, C. Gumus, and T. Ozpozan, Mater. Chem. Phys. 129, 183 (2011).
S. Thanikaikarasan, C. Vedhi, X.S. Shajan, and T. Mahalingam, Solid State Sci. 15, 142 (2013).
T. Ben Nasr, N. Kamoun, M. Kanzari, and R. Bennaceur, Thin Solid Films 500, 4 (2006).
A. Kassim, W.T. Tan, S.M. Ho, and N. Saravanan, Appl. Sci. Eng. 11, 17 (2010).
S.B. Quadri, E.F. Skelton, D. Hsu, A.D. Dinsmore, J. Yang, H.F. Gray, and B.R. Ratna, Phys. Rev. B 60, 9191 (1999).
S. Thanikaikarasan, T. Mahalingam, M. Raja, T. Kim, and Y.D. Kim, J. Mater. Sci.: Mater. Electron. 20, 727 (2009).
K.R. Murali, A. Austine, and D.C. Trivedi, Mater. Lett. 59, 2621 (2005).
T. Yodo, R. Ueda, K. Morio, R. Yamasita, and S. Tanaka, J. Appl. Phys. 68, 3212 (1990).
F. Sakurai, K. Suto, S. Sanda, and J. Nishizawa, J. Electrochem. Soc. 149, G100 (2002).
Acknowledgements
One of the authors acknowledges the UGC for financial assistance under FDP. The authors are grateful to STIC-CUSAT for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deepa, K., Dhanya, A.C. & Remadevi, T.L. Investigations on the Structure, Morphology, and Optoelectronic Properties of Chemically Deposited ZnSe Thin Films: The Effect of Solution pH. J. Electron. Mater. 43, 3155–3161 (2014). https://doi.org/10.1007/s11664-014-3229-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-014-3229-8