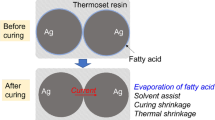
A homogeneous electrically conductive silver paste without solid or particle phase was developed using silver alkylcarbamates [(C n H2n−1NHCOO)2Ag, n ≤ 4] as the precursor of the functional phase. The silver alkylcarbamates were light insensitive and had a low decomposition temperature (below 200°C). The paste was a non-Newtonian fluid with viscosity significantly depending on the content of the thickening agent ethyl cellulose. Array patterns with a resolution of 20 μm were obtained using this paste by a micropen direct-writing method. After the paste with about 48 wt.% silver methylcarbamate [(CH3NHCOO)2Ag] precursor was sintered at 180°C for 15 min, an electrically conductive network consisting of more than 95 wt.% silver was formed, and was found to have a volume electrical resistivity on the order of 10−5 Ω cm and a sheet electrical resistivity on the order of 10−2–10−3 Ω/□. The cohesion strength within the sintered paste and the adhesion strength between the sintered paste layer and the alumina ceramic substrate were tested according to test method B of the American Society for Testing and Materials standard D3359-08. None of the sintered paste layer was detached under the test conditions, and the cohesion and adhesion strengths met the highest grade according to the standard.
Similar content being viewed by others
References
K.J. Lee, B.H. Jun, J. Choi, Y. Lee II, J. Joung, and Y.S. Oh, Nanotechnology 18, 335601 (2007).
J.G. Bai, J.N. Calata, and G.Q. Lu, IEEE Trans. Electron. Packag. Manuf. 30, 241 (2007).
S.H. Park, D.S. Seo, and J.K. Lee, Colloids Surf. A Physicochem. Eng. Asp. 313–314, 351 (2008).
H.H. Lee, K.S. Chou, and Z.W. Shih, Int. J. Adhes. Adhes. 25, 437 (2005).
T. Kasuga, K. Shimoda, and M. Yamakawa, U.S. patent 7198736B2 (2007).
M.N. Enguyan and M.A. Block, CN patent 1055164A (1991).
Y.M. Shan, CN patent 1136208A (1996).
A.J. Slifka and E.S. Drexler, J. Electron. Mater. 31, 286 (2002).
H.C. Jung, S.H. Cho, J.W. Joung, and Y.S. Oh, J. Electron. Mater. 36, 1211 (2007).
R.W. Vest, Am. Ceram. Soc. Bull. 65, 631 (1986).
C.J. Ting, C.S. His, and H.Y. Lu, J. Am. Ceram. Soc. 83, 2945 (2000).
Y. Takashi and L. Kazuhiko, J. Am. Ceram. Soc. 73, 1953 (1990).
W.F. Linke, Solubilities of Inorganic and Metal Organic Compounds, Vol. 1 (Washington, DC: American Chemical Society, 1958), pp. 5–147.
J.C. Emeleus, S.R. Nyholm, and A.F. Trotman-Dickenson, Comprehensive Inorganic Chemistry, Vol. 3 (Oxford: Pergamon, 1973), pp. 79–128.
F.A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry (New York: Wiley-Interscience, 1962), pp. 862–867.
E. Szlyk, P. Piszczek, A. Grodzicki, M. Chaberski, A. Golinski, J. Szatkowski, and T. Blaszczyk, Chem. Vap. Deposition 7, 111 (2001).
K.M. Chi, K.H. Chen, S.M. Peng, and G.H. Lee, Organometallics 15, 2575 (1996).
K.M. Chi and Y.H. Lu, Chem. Vap. Deposition 7, 116 (2001).
K. Binnemans, R. Van Deun, B. Thijs, I. Vanwelkenhuysen, and I. Geuens, Chem. Mater. 16, 2021 (2004).
D.R. Whitcomb and R. Rogers, J. Chem. Crystallogr. 26, 99 (1996).
H.A. Chiong and O. Daugulis, Organometallics 25, 4054 (2006).
M. George and R.G. Weiss, J. Am. Chem. Soc. 123, 10393 (2001).
H.B. Wright and M.B. Moore, J. Org. Chem. 70, 3865 (1948).
E. Katchalski, C. Berliner-Klibanski, and A. Berger, J. Org. Chem. 73, 1829 (1951).
C.W. Hoerr, H.J. Harwood, and A.W. Ralston, J. Org. Chem. 9, 201 (1944).
S.H. Park, D.S. Seo, and J.K. Lee, Colloids Surf. A Physicochem. Eng. Asp. 313–314, 197 (2008).
I.Y. Kim, Y.A. Song, H.C. Jung, J.W. Joung, S.S. Ryu, and J.R. Kim, J. Electron. Mater. 37, 1863 (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Li, X., Wang, X. et al. Electrically Conductive Thick Film Made from Silver Alkylcarbamates. J. Electron. Mater. 39, 2267–2273 (2010). https://doi.org/10.1007/s11664-010-1327-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-010-1327-9