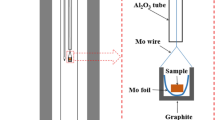
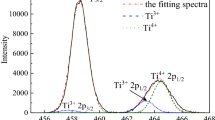
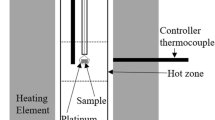
Abstract
Knowledge of the equilibrium phase relationship in the V2O5–Na2O system is critical and essential for the sodium salts roasting process of slag-bearing vanadium, and those fundamental studies can be used to accelerate industrial optimization and update the process. Therefore, phase equilibrium and quenching and thermal analysis experiments on the V2O5–Na2O binary system were carried out in the present work under pO2 of 10−3.5 atm. The V2O5–Na2O system has 7 primary phase fields including two simple oxides (V2O5 and Na2O), and 5 binary compounds (Na2V12O31, Na10V24O65, NaVO3, Na4V2O7, and Na3VO4). The existence of Na2V12O31 and Na10V24O65 was experimentally confirmed and then characterized by EPMA, no extensive compounds or solid solutions were detected in the present study. The liquidus lines were experimentally determined in most of the primary phase fields in the temperature range from 500 °C to 800 °C. The temperatures and components of some invariant reactions, including Liq → V2O5 + Na2V12O31 (640 °C, 53 mol pct Na2O), Liq → Na10V24O65 + NaVO3 (504 °C, 40.2 mol pct Na2O), Liq → NaVO3 + Na4V2O7 (540 °C, 59.0 mol pct Na2O), and a new peritectic reaction at 570 °C with 32 mol pct Na2O were rechecked, and Liq + Na2V12O31 → Na10V24O65 was also reconstructed. The large amount of new phase equilibrium data proposed in this work will serve as reliable input for future thermodynamic reassessments of the V2O5–Na2O system within the CALPHAD framework to extensively improve the current V2O5-based thermodynamic databases.
Graphical Abstract








Similar content being viewed by others
References
M. Petranikova, A.H. Tkaczyk, A. Bartl, A. Amato, and C. Tunsu: Waste Manage., 2020, vol. 113, pp. 521–44.
R. Gilligan and A.N. Nikoloski: Miner. Eng., 2019, https://doi.org/10.1016/j.mineng.2019.106106.
J. Shi, M. Chen, X. Wan, P. Taskinen, and A. Jokilaakso: JOM, 2020, vol. 72, pp. 3204–12.
G. Pei, J. Xiang, X. Lv, G. Li, Wu. Shanshan, D. Zhong, and W. Lv: J. Alloys Compd., 2019, vol. 794, pp. 465–72.
W. Xie, X. Xing, and Z. Cao: J. Am. Ceram. Soc., 2020, vol. 103, pp. 5312–24.
Y. Chen, M. Wang, Lu. Songle, Tu. Jiguo, and S. Jiao: Electrochim. Acta, 2020, vol. 331, pp. 1–9.
M. Chandra, T.S. Khan, R. Shukla, S. Ahamad, A. Gupta, S. Basu, M. Ali Haider, and R.S. Dhaka: Electrochimica Acta, 2020, vol. 331, pp. 1–10.
B. Su, H. Liang, J. Liu, J. Wu, N. Sharma, Q. Gu, B. Johannessen, and D.Y.W. Yu: Chem. Commun., 2020, vol. 56, pp. 8245–48.
I.-H. Jung and M.-A. Van Ende: Metall. Mater. Trans. B., 2020, vol. 51B, pp. 1851–74.
G. Pei, J. Xiang, L. Yang, X. Jin, and X. Lv: Calphad, 2020, vol. 70, pp. 1–6.
H. Sorum and H. Flood: Tidsskrift for Kjemi Bergvesen og Metallurgi, 1943, vol. 5, pp. 55–59.
R.P. Ozerov, V.V. Illarionov, and E.V. Kildildisheva: Zhurnal Neorganicheskoi Khimii, 1957, vol. 2, pp. 883–9.
M.P. Glazyrin and A.A. Fotiev: Izvestiya Akademii Nauk SSSR, Neorganicheskie Materialy, 1968, vol. 4, pp. 82–7.
G.A. Kolta and I.F. Hewaidy: Thermochim. Acta, 1972, vol. 25(7), pp. 327–30.
V. Danek, K. Matiasovsky, and J. Balajka: Chemicke Zvesti, 1973, vol. 27, pp. 748–51.
R.C. Kerby and J.R. Wilson: Can. J. Chem., 1973, vol. 51, pp. 1032–40.
A.A. Fotiev, V.L. Volkov, and N.K. Valikhanova: Zh. Neorg. Khim., 1975, vol. 2, pp. 497–500.
M.P. Glazyrin and A.A. Fotiey: Izvestiya Akademii Nauk SSSR Neorg. Mater., 1968, vol. 4, pp. 82–87.
J.R. Wilson and R.C. Kerby: Can. J. Chem., 1973, vol. 51, pp. 1032–40.
B.G. Golovkin, L.V. Kristallov, and M.V. Kruchinina: Zhurnal Neorganicheskoj Khimii, 1995, vol. 40, pp. 514–18.
J. Lee, S.Y. Kwon, and I.-H. Jung: J. Eur. Ceram. Soc., 2021, vol. 41, pp. 7946–56.
V. Danek, I. Votava, K. Matiasovsky, and J. Balajka: Chemicke Zvesti, 1974, vol. 28, pp. 728–32.
Acknowledgments
This work was supported by the National Key R&D Program of China (2018YFC1900500), National Natural Science Foundation of China (51904048), and the Graduate Scientific Research and Innovation Foundation of Chongqing, China (Grant No. CYB20002).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lv, X., Pei, G., Zhong, D. et al. Phase Equilibrium of the V2O5–Na2O System. Metall Mater Trans B 53, 2695–2703 (2022). https://doi.org/10.1007/s11663-022-02560-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02560-z