Abstract
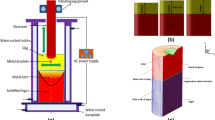
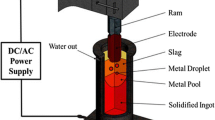
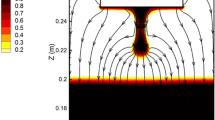
The desulfurization behavior in the electromagnetically controlled vibrating-electrode electroslag remelting (ESR) furnace was analyzed, modeled, and numerically simulated. A new transient fully coupled mathematical model with the magneto-hydro-dynamic (MHD) multiphase method based on the dynamic mesh-based approach has been developed. Results demonstrate that horizontally vibrating electrode in the slag results in the highest sulfur content contrary to vertically vibrating electrode. The maximum removal ratio of sulfur with horizontally vibrating electrode can reach up to 78 pct, which is higher than in the case of other vibration modes. Furthermore, sensitivity analysis with respect to the applied current, frequency, filling ratio, slag thickness, and insertion depth was performed. Calculated maximum sulfur content along the slag diameter decreased from 0.0754 to 0.0543 pct when the applied current increased from 1200 to 1800 A. The maximum sulfur concentration in the slag was reached at the frequency of 0.25 Hz and it increased with the increase in the filling ratio from 0.38 to 0.54. On the other hand, when the thickness of the slag pool decreased, the maximum sulfur concentration also decreased from 0.0812 to 0.0473 pct. With the increase in insertion depth from 2.5 to 7.5 mm, the maximum mass fraction of sulfur in slag increased by 5 pct.
















Similar content being viewed by others
Abbreviations
- a [i] :
-
Activity of element i in the metal
- a (i) :
-
Activity of element i in the slag
- \(\mathop{A}\limits^{\rightharpoonup}\) :
-
Electric current (A)
- \(\mathop{B}\limits^{\rightharpoonup}\) :
-
Magnetic flux density (T)
- c p :
-
Specific heat of mixture phase (J/(kg·K))
- c p,m :
-
Specific heat of metal phase (J/(kg·K))
- c p,s :
-
Specific heat of slag phase (J/(kg·K))
- \(C_{{{\rm S}^{{2 - }} }}\) :
-
Sulfide capacity of the slag
- \(e_{i}^{j}\) :
-
Interaction coefficient of element j on solvent i
- \(\mathop{E}\limits^{\rightharpoonup}\) :
-
Electric field intensity (N/C)
- \(f_{{\left[ i \right]}}\) :
-
Activity coefficient of the element in the metal
- f vib :
-
The number of vibration of electrode per minute(c/min)
- \(\mathop{F}\limits^{\rightharpoonup}_{{\rm force}}\) :
-
Lorentz force (N/m3)
- \(\mathop{F}\limits^{\rightharpoonup}_{{\rm tbf}}\) :
-
Thermal buoyancy force (N/m3)
- \(\mathop{F}\limits^{\rightharpoonup}_{{\rm sbf}}\) :
-
Solutal buoyancy force (N/m3)
- \(\mathop{F}\limits^{\rightharpoonup}_{{\rm st}}\) :
-
Damping force (N/m3)
- \(\mathop{H}\limits^{\rightharpoonup}\) :
-
Magnetic field intensity (A/m)
- \(\mathop{J}\limits^{\rightharpoonup}\) :
-
Current density (A/m2)
- \(\left|\mathop{J}\limits^{\rightharpoonup} \right|\) :
-
Local current density (A/m2).
- k eff :
-
Effective thermal conductivity (W/(m·K))
- K :
-
Reaction equilibrium constant
- L :
-
Latent heat of slag (J/kg)
- L i :
-
Sulfur partition ratio
- \(\dot{m}\) :
-
Melting rate (kg/s)
- n s :
-
Molar mass of sulfur
- \(P_{{{\rm O}_{2} }},\;P_{{{\rm S}_{2} }}\) :
-
Pressure of O2 and S2 (Pa)
- \(\mathop{P}\limits^{\rightharpoonup}\) :
-
Pressure (Pa)
- Q :
-
Joule heating (W/m3)
- Q t :
-
Total value of Joule heating (W/m3)
- r i :
-
Volume fraction of steel or slag phase
- \(S_{\phi }\) :
-
Source term of Eq.(10)
- \(S_{\varphi }\) :
-
Source term of Eq.(18)
- S 1st :
-
Mass transfer rate caused by thermochemical reaction
- S 2nd :
-
Mass transfer rate caused by electrochemical reaction
- t :
-
Time (s)
- T :
-
Temperature (K)
- \(\mathop{v}\limits^{\rightharpoonup}\) :
-
Velocity (m/s)
- \(\mathop{v}\limits^{\rightharpoonup} _{\rm g}\) :
-
Motion velocity of the moving mesh(m/s)
- V A :
-
Volume of slag layer(m3)
- W :
-
Internal energy of mixture phase(J/m3
- \(\gamma _{{\rm metal}}\) :
-
Mass transfer coefficient of sulfur in the metal (m/s)
- \(\gamma _{{\rm slag}}\) :
-
Mass transfer coefficient of sulfur in the slag (m/s)
- ζ :
-
Properties of mixture phase
- ζ m :
-
Properties of metal phase
- ζ s :
-
Properties of slag phase
- θ :
-
Diffusion coefficient
- \(\lambda [i],\;\lambda (i)\) :
-
Mass fraction of sulfur in the metal and the slag
- \(\lambda _{{\left( {{\text{Al}}_{{\text{2}}} {\text{O}}_{{\text{3}}} } \right)}},\;\lambda _{{\left( {{\text{FeO}}} \right)}},\;\lambda _{{\left( {{\text{SiO}}_{{\text{2}}} } \right)}}\) :
-
Mass fraction of aluminum oxide, ferrous oxide, and silicon dioxide in the slag
- \(\mu\) :
-
Dynamic viscosity (Pa·s)
- μ eff :
-
Effective viscosity (Pa·s)
- ρ :
-
Density (kg/m3)
- \(\bar{\rho}\) :
-
Density of mixture phase (kg/m3)
- \(\rho _{i}\) :
-
Density of steel or slag phase
- \(\rho _{\rm m}\) :
-
Density of metal phase (kg/m3)
- \(\rho _{rm s}\) :
-
Density of slag phase (kg/m3)
- σ :
-
Electrical conductivity (1/(Ω·m))
- φ :
-
Dissolver content
- \(\phi\) :
-
Electric potential (V)
- ψ i :
-
Mass transfer coefficient of sulfur
- ψ slag, ψ metal :
-
Effective mass transfer coefficient of sulfur in molten slag and metal
- q :
-
The number of electrons during the reaction
- \(\varpi _{p}\) :
-
Coefficient of power efficiency
- \(\Gamma _{\varphi}\) :
-
Diffusion coefficient of sulfur (m2/s)
- \(\Lambda\) :
-
Optical basicity of slag
- α :
-
The volume fraction of metal
References
A. Kharicha, E. Karimi-Sibaki, M. Wu, A. Ludwig, and J. Bohacek: Steel Res. Int., 2018, vol. 89(1), p. 1700100.
M. Kato, K. Hasegawa, S. Nomura, and M. Inouye: Trans. ISIJ., 1983, vol. 23(7), pp. 618–27.
J.H. Wei and A. Mitchell: Acta Metall. Sin., 1984, vol. 20(5), pp. 280–7.
Z.B. Li: Electroslag Metallurgy Theory and Practice, Metallurgical Industry Press, Beijing, 2010.
F. Wang, Y.C. Lou, R. Chen, Z.W. Song, and B.K. Li: China Found., 2015, vol. 12(4), pp. 285–92.
F. Wang, Y.L. Xiong, and B.K. Li: Steel Res. Int., 2019, vol. 90(4), p. 1800092.
F. Wang, Q. Wang, and B.K. Li: ISIJ Int., 2017, vol. 57(1), pp. 91–9.
M. Hugo, B. Ussoubs, A. Jardy, J. Escaffre, and H. Poisson: Metall. Mater. Trans. B., 2016, vol. 47B(8), pp. 2607–22.
J. Yanke, K. Fezi, R.W. Trice, and M.J.M. Krane: Numer. Heat Trans. A Appl., 2015, vol. 67, pp. 268–92.
K. Fezi, J. Yanke, and M.J.M. Krane: Metall. Mater. Trans. B., 2015, vol. 46B(2), pp. 766–79.
E. Karimi-Sibaki, A. Kharicha, J. Bohacek, M. Wu, and A. Ludwig: Metall. Mater. Trans. B., 2015, vol. 46B(5), pp. 2049–61.
L. Jonsson, D. Sichen, and P. Jönsson: ISIJ Int., 1998, vol. 38(3), pp. 260–67.
X. Yang, J. Jiao, R. Ding, C. Sh, and H. Guo: ISIJ Int., 2009, vol. 49(12), pp. 1828–37.
C.B. Shi, X.C. Chen, H.J. Guo, Z.J. Zhu, and H. Ren: Steel Res. Int., 2012, vol. 83(5), pp. 472–86.
D. Hou, Z. Jiang, Y. Dong, Y. Li, W. Gong, and F. Liu: Metall. Mater. Trans. B., 2017, vol. 48B(6), pp. 1885–98.
Q. Wang, Z. He, G. Li, B. Li, C. Zhu, and P. Chen: Int. J. Heat Mass Transf., 2017, vol. 104, pp. 943–51.
N.Q. Minh and T.B. King: Metall. Trans. B., 1979, vol. 10(6), pp. 623–29.
U. Mitra and T.W. Eagar: Metall. Trans. A., 1984, vol. 15A(1), pp. 217–27.
A.H. Dilawari and J. Szekely: Metall. Mater. Trans. B., 1978, vol. 9B, pp. 77–87.
A. Ludwig, A. Kharicha, and M. Wu: JOM., 2014, vol. 68(45), pp. 2191–97.
A.H. Dilawari and J. Szekely: Metall. Trans. B., 1977, vol. 8B, pp. 227–36.
A. Jardy, D. Ablitzer, and J.F. Wadier: Metall. Trans. B., 1991, vol. 22B, pp. 111–20.
O. Biro and K. Preis: IEEE Trans. Magn., 1989, vol. 25, pp. 3145–59.
A. Mitchell: Sci. Eng. A., 2005, vol. 10, pp. 413–14.
A. Weber, A. Jardy, B. Dussoubs, D. Ablitzer, S. Ryberon, V. Schmitt, S. Hans, and H. Poisson: Metall. Mater. Trans. B., 2009, vol. 40B, pp. 271–80.
X. Huang, B. Li, and Z. Liu: Int. J. Heat Mass Transf., 2017, vol. 104, pp. 943–51.
G. Hoyle: Muscles and Their Neural Control, Wiley, New York, 1983.
A. Mitchell and S. Joshi: Metall. Trans., 1973, vol. 4, pp. 631–42.
P.G. Jönsson and L.T.I. Jonsson: ISIJ Int., 2001, vol. 41(11), pp. 1289–302.
Z.Q. Liu, R. Niu, Y.D. Wu, B.K. Li, Y. Gan, and M.H. Wu: Int. J. Heat Mass Transf., 2021, vol. 173, p. 121237.
W.T. Lou and M.Y. Zhu: Metall. Mater. Trans. B., 2014, vol. 45B(5), pp. 1706–22.
C.P. Manning and R.J. Fruehan: Metall. Mater. Trans. B., 2013, vol. 44B(1), pp. 37–44.
J.C. Lamont and D.S. Scott: AIChE J., 1970, vol. 16(37), pp. 513–19.
B.D. Prasher and G.B. Wills: Ind. Eng. Chem. Process Des. Dev., 1973, vol. 12, pp. 351–54.
Y. Kawase, B. Halard, and M. Moo-Young: Eng. Sci., 1987, vol. 42, pp. 1609–17.
A. Shankar, M. Gornerup, A.K. Lahiri, and S. Seetharaman: Metall. Mater. Trans. B., 2006, vol. 37B, pp. 941–47.
Z.H. Jiang, D. Hou, Y.W. Dong, Y.L. Cao, H.B. Cao, and W. Gong: Metall. Mater. Trans. B., 2016, vol. 47B(2), pp. 1465–74.
A. Karasev and H. Suito: Metall. Mater. Trans. B., 1999, vol. 20B(2), pp. 249–57.
T. Yoshikawa and K. Morita: Metall. Mater. Trans. B., 2007, vol. 38B(4), pp. 671–80.
A.J. Bard and L.R. Faulkner: Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 2001, pp. 22–38.
F. Wang, Q. Wang, J. Baleta, and B. Li: JOM., 2019, vol. 71(11), pp. 4198–4205.
Acknowledgments
The authors appreciate the financial support by the National Natural Science Foundation of China (No. 52171031), the Fundamental Research Funds for the Central Universities (Nos. N2125039 and N2025020), and the National Science and Technology Major Projects (Nos. 2017-VI-0015-0087 and 2017-VI-0018-0090).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, F., Tan, J., Huang, X. et al. Mathematical and Numerical Predictions of Desulfurization Behavior in the Electromagnetically Controlled Vibrating-Electrode Electroslag Remelting Furnace. Metall Mater Trans B 53, 1792–1805 (2022). https://doi.org/10.1007/s11663-022-02487-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02487-5