Abstract
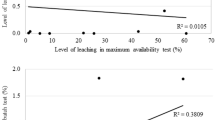
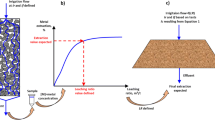
In this study, an optimal sampling schedule was developed for leaching experiments with the objective of improving the confidence of the kinetic parameters. This study shows that there is an improvement in the confidence interval from uniform sampling and the method presented here. The optimal sampling times were determined by reducing the determinant of the covariance matrix associated with the kinetic constant, which can be expressed through the covariance matrix of the extracted fraction, \(X,\) used to generate a function to distribute the sampling times in the experiment. The method presented here requires minimal knowledge a priori of the system to be characterized. Only the kinetic expression for the system is required. The methodology was applied to a simulated case and experimental case study of ammoniacal leaching of copper slags. The simulations conducted indicated a lower value of the standard deviation of 1.40·10−4 min−1 for optimized sampling times and a value of 1.78·10−4 min−1 for uniform distribution. The experimental validation results indicated a reduction of the coefficient of variation for optimized experiments of 9.3 pct (less uncertainty) from 29.7 pct (uniform sampling) to 14.6 pct (optimized sampling). Thus, the methodology proposed here is successful in decreasing the uncertainty in laboratory leaching experiments.



Similar content being viewed by others
References
F. Habashi: Hydrometallurgy., 2005, vol. 79(1–2), pp. 15–22.
R. Bartlett: Solution Mining 2e, Routledge, 2013.
Rees, K. L. (2000). The leaching and adsorption behaviour of gold ores. PhD thesis, Department of Chemical Engineering, The University of Melbourne.
F. Arroyo and C. Fernandez-Pereira: Ind. Eng. Chem. Res., 2008, vol. 47(9), pp. 3186–91.
F. Arroyo, C. Fernández-Pereira, and P. Bermejo: Minerals., 2015, vol. 5(2), pp. 298–313.
F.K. Crundwell: Hydrometallurgy., 2013, vol. 139, pp. 132–48.
F.K. Crundwell: Hydrometallurgy., 1995, vol. 39(1–3), pp. 321–35.
S. Vyazovkin and C.A. Wight: Annu. Rev. Phys. Chem., 1997, vol. 48(1), pp. 125–49.
H.Y. Sohn: Metall. Trans. B., 1978, vol. 9(1), pp. 89–96.
Brown, M., Dollimore, D., & Galwey, A. (1980). Comprehensive Chemical Kinetics (Vol. 22). Elsevier
Faraji, F., Alizadeh, A., Rashchi, F., & Mostoufi, N.: Reviews in Chemical Engineering. 2020, pp. 000010151520190073
W. Astuti, T. Hirajima, T. Sasaki, and N. Okibe: Miner. Metall. Process., 2015, vol. 32(3), pp. 176–85.
C. Thubakgale, R. Mbaya, and K. Kabongo: Int. J. Chem. Mol. Nucl. Mater. Metall. Eng., 2012, vol. 2012, pp. 228–32.
M.C. Fuerstenau and K.N. Han: Principles of Mineral Processing, Society for Mining, Metallurgy, and Exploration Inc, Colorado, 2009.
O. Levenspiel: Chemical Reaction Engineering, Wiley, 1999.
D.Z. D’Argenio: J. Pharmacokinet. Biopharm., 1981, vol. 9(6), pp. 739–56.
G. Veloso, R. Simpson, H. Núñez, C. Ramírez, S. Almonacid, and A. Jaques: J. Food Eng., 2021, vol. 306, p. 110610.
A.V. Jaques, M.B. Barraza, and J.C. Lacombe: J. Phase Equilib. Diffus., 2015, vol. 36(1), pp. 22–27.
C.P. Kitsos: Optimal Experimental Design for Non-Linear Models: Theory and Applications, Springer, Berlín, 2013.
P. Coursol, P.J. Mackey, and C.M. Diaz: Proc. Copper., 2010, vol. 2, pp. 649–68.
A. Aracena, E. Rodríguez, and O. Jerez: Hydrometallurgy., 2020, vol. 192, p. 105290.
A. Aracena, A. Valencia, and O. Jerez: Metals., 2020, vol. 10(6), p. 712.
M. Arzutug, M. Kocakerim, and M. Copur: Ind. Eng. Chem. Res., 2004, vol. 43(15), pp. 4118–23.
Guo-dong, Z., & Qing, L.: International Conference on Chemistry and Chemical Engineering (ICCCE), 2010, 216-20.
D. Li, C. Wang, Y. Chen, and X. Jie: Chin. J. Power Sources., 2009, vol. 33, pp. 454–57.
Z. Liu, Z. Yin, S. Xiong, Y. Chen, and Q. Chen: Hydrometallurgy., 2014, vol. 144–145, pp. 86–90.
Y. Huang, Z. Yin, Z. Ding, J. Feng, and L. Chunxia: Hydrometallurgy., 2018, vol. 179, pp. 198–206.
O. Furman, A. Teel, and R. Watts: Environ. Sci. Technol., 2010, vol. 44(16), pp. 6423–28.
A. Ekmekyapar, R. Oya, and A. Kunkul: Chem. Biochem. Eng. Q., 2003, vol. 17(4), pp. 261–26.
A. Aracena, Y. Vivar, O. Jerez, and D. Vásquez: Miner. Process. Extr. Metall. Rev., 2015, vol. 36(5), pp. 317–23.
L. Beckstead and J. Miller: Metall. Trans., 1977, vol. 8(1), pp. 19–29.
Radmehr, V., Koleini, S., Khalesi, M., & Tayakoli, M.: J. Inst. Eng. (India), 2013, 94(2), 95-104.
R.H. Myers, D.C. Montgomery, G. Geoffrey, and T.J. Robinson: Generalized Linear Models With Applications in Engineering and the Sciences, Wiley, New Jersey, 2010.
Acknowledgments
The authors are grateful for the support of ANID, via “FONDECYT INICIACIÓN, 11180432 project”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ojeda, C., Jaques, A. & Aracena, A. Optimal Sampling Times for Leaching Experiments. Metall Mater Trans B 53, 1082–1088 (2022). https://doi.org/10.1007/s11663-022-02426-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02426-4