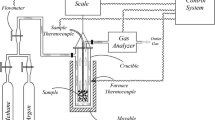
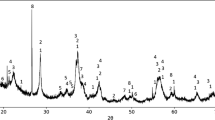
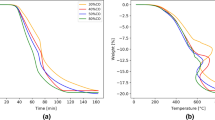
Abstract
Carburization of Comilog ore, pure MnO powder, and MnO pellet by natural gas was studied by experimental work. XRD, SEM-EDS, and thermogravimetric techniques were used for characterization and reduction behavior analysis. It was found that manganese carbide is formed through direct carburization by methane without methane cracking, and the process was governed by a diffusion-controlled reaction. Even low quantities of some phases in the manganese sources could affect the reduction process adversely. Although increase in temperature intensified the reduction severity, the formation of manganese silicate network at high temperatures hindered the reduction progress of Comilog ore; the best carburization results were achieved at 1100 °C with 24 pct manganese carbide formation. The detrimental effect of aluminates on the gaseous reduction was through lowering the melting temperature of manganese silicates. However, gaseous reduction by methane was adversely affected more by a non-reducible (Mn,Ca)O phase evolved during the pelletizing process on the pore walls rather than by sintering and formation of manganese silicate network. Although neither sintering nor non-reducible phase on the pore walls was observed in the reduction of MnO powder, no complete carburization was occurred. Soot formation and no gas access to the particle surface were recognized as the signs of blocked reduction progress.
Graphical Abstract























Similar content being viewed by others
References
S.E. Olsen, M. Tangstad, and T. Lindstad: Production of Manganese Ferroalloys, Tapir Academic Press, Trondheim, 2007.
G. Akdogan and R.H. Eric: Metall. Mater. Trans. B., 1995, vol. 26B(1), pp. 13–24.
K. Berg and S. Olsen: Metall. Mater. Trans. B., 2000, vol. 31B(3), pp. 477–90.
R. Kononov, O. Ostrovski, and S. Ganguly: Metall. Mater. Trans. B., 2008, vol. 39B(5), pp. 662–8.
A. Cheraghi, H. Yoozbashizadeh, and J. Safarian: Miner. Process. Extr. Metall. Rev., 2020, vol. 41(3), pp. 198–215.
N. Anacleto, O. Ostrovski, and S. Ganguly: ISIJ Int., 2004, vol. 44(10), pp. 1615–22.
N. Anacleto, O. Ostrovski, and S. Ganguly: ISIJ Int., 2004, vol. 44(9), pp. 1480–7.
A. Bhalla and R.H. Eric: Infacon XIV., 2015, vol. 1, pp. 461–9.
K. Ohla and H. Grabke: Mater. Corros., 1982, vol. 33(6), pp. 341–6.
Aripin H, Priatna E, Busaeri N, Hiron N, Sabchevski S: IOP Conference Series: Materials Science and Engineering, 2019, pp. 012036.
B. Liu, Y. Zhang, Z. Su, Z. Peng, G. Li, and T. Jiang: JOM., 2017, vol. 69(9), pp. 1669–75.
T. Zhang and M.D. Amiridis: Appl. Catal. A., 1998, vol. 167(2), pp. 161–72.
H. Dalaker and P. Tetlie: Celebrating the Megascale, Springer, Cham, 2014, pp. 537–46.
O. Ostrovski and G. Zhang: AIChE J., 2006, vol. 52(1), pp. 300–10.
Ostrovski O, Yastreboff M, Johnston RF, Anacleto N, Ganguly S, inventors; Unisearch Limited, Sydney (AU); Temco Pty LTD’ Bnsbane (AU), assignee. Solid state reduction of oxides. US2004.
O. Ostrovski: Celebrating the Megascale, Springer, Cham, 2014, pp. 529–36.
K.S. Go, S.R. Son, S.D. Kim, K.S. Kang, and C.S. Park: Int. J. Hydrogen Energy., 2009, vol. 34(3), pp. 1301–9.
M. Rydén and A. Lyngfelt: Int. J. Hydrogen Energy., 2006, vol. 31(10), pp. 1271–83.
Elliott R, Barati M: Extraction 2018, Springer, 2018, pp. 1129–40.
B. Liu, Y. Zhang, Z. Su, M. Lu, G. Li, and T. Jiang: Powder Technol., 2018, vol. 325, pp. 271–9.
A. Cheraghi, H. Yoozbashizadeh, and J. Safarian: INFACON XV., 2018, vol. 1, pp. 157–67.
A. Cheraghi, H. Yoozbashizadeh, E. Ringdalen, and J. Safarian: Metall. Mater. Trans. B., 2019, vol. 50B, pp. 1566–80.
E. Turkdogan and J. Vinters: Metall. Mater. Trans. B., 1971, vol. 2B(11), pp. 3175–88.
Y. Gao, M. Olivas-Martinez, H. Sohn, H.G. Kim, and C.W. Kim: Metall. Mater. Trans. B., 2012, vol. 43B(6), pp. 1465–75.
M. Tangstad, P. Calvert, H. Brun, and A. Lindseth: Infacon X., 2004, vol. 1, pp. 213–22.
Y.-B. Kang, I.-H. Jung, S.A. Decterov, A.D. Pelton, and H.-G. Lee: ISIJ Int., 2004, vol. 44(6), pp. 965–74.
E. Serris, L. Favergeon, M. Pijolat, M. Soustelle, P. Nortier, R. Gärtner, et al.: Cem. Concr. Res., 2011, vol. 41(10), pp. 1078–84.
A. Cheraghi, H. Becker, H. Eftekhari, H. Yoozbashizadeh, and J. Safarian: Mater. Today Commun., 2020, vol. 25, p. 101382.
M. Wilson, M. Berrow, and W. McHardy: Miner. Mag., 1970, vol. 37(289), pp. 618–23.
B. Sorensen, S. Gaal, E. Ringdalen, M. Tangstad, R. Kononov, and O. Ostrovski: Int. J. Miner. Process., 2010, vol. 94(3), pp. 101–10.
Y.-B. Kang, H.S. Kim, J. Zhang, and H.-G. Lee: J. Phys. Chem. Solids., 2005, vol. 66(2–4), pp. 219–25.
D. Friedmann, A. Pophanken, and B. Friedrich: Journal of Sustainable Metallurgy., 2017, vol. 3(2), pp. 219–29.
R. Snow: J. Am. Ceram. Soc., 1943, vol. 26(1), pp. 11–20.
Acknowledgment
This study has been done in the laboratories at NTNU which is acknowledged. The current work has been supported by the Research Domain 2 in SFI-Metal production; a Norwegian Centre for Research-Based Innovation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted May 17, 2021; accepted November 20, 2021.
Rights and permissions
About this article
Cite this article
Cheraghi, A., Yoozbashizadeh, H. & Safarian, J. Carburization of Manganese Oxide Sources by Natural Gas. Metall Mater Trans B 53, 744–759 (2022). https://doi.org/10.1007/s11663-021-02398-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02398-x