Abstract
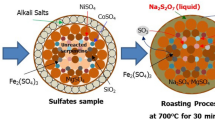
Nickel is mostly extracted from sulfide ores, however, laterite ores account for over 60 pct of all nickel resources in the world, and despite its predominance, there is no well-established process to extract nickel from such ores. Nickel in laterites is hosted in many different compounds such as oxides, hydroxides, and silicates minerals. The sulfation-roasting-leaching process has the potential to change this scenario once it can be applied to all kinds of nickel laterite ores and does not consume much acid, as in the atmospheric leaching process. The main characteristic of the process is the iron sulfates decomposition during roasting steps, which produces sulfur trioxide (SO3). The sulfur trioxide is reactive with metals such as nickel and cobalt, converting them to soluble sulfates, and reducing acid consumption. Experiments were conducted to establish the optimal conditions to extract nickel from laterite ores using the sulfation-roasting-leaching process. Various parameters were investigated: water addition, sulfuric acid concentration, the number of heat-treatments steps, roasting temperature and time, leaching time, and solid/liquid ratio. Furthermore, the phase changes during thermal treatments were investigated to identify the mechanisms involved in the transformation of the minerals. Experimental results indicated that nickel forms sulfates through three different ways: reacting with H2SO4 during sulfation, with Fe2(SO4)3 (ferric sulfate) or Fe(OH)SO4 (basic iron sulfates) during the heat-treatments, and also throughout the leaching step due to iron-rich phase dissolution. More than 83.0 pct Ni, 90.0 pct Co, 61.3 pct Al, 17.3 pct Ca, 85.7 pct Mg, 87.5 pct Mn, 1.1 pct Ti, and 16.6 pct Fe were extracted under optimums conditions.
Graphic Abstract






















Similar content being viewed by others
References
F.K. Crundwell, M.S. Moats, V. Ramachandran, T.G. Robinson, and W.G. Davenport: in Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals, Elsevier, 2011, pp. 21–37.
V.R.C. Thanu, C. Andrew, and M. Jayakumar: Surfaces and Interfaces, 2020, vol. 19, p. 100539.
X.Y. Guo, W.T. Shi, D. Li, and Q.H. Tian: Trans. Nonferrous Met. Soc. China, 2011, vol. 21, pp. 191–5.
D.D. Radev: Adv. Powder Technol., 2010, vol. 21, pp. 477–82.
S. Geng, H. Dong, Y. Lu, S. Wang, Y. Huang, X. Zou, Y. Zhang, Q. Xu, and X. Lu: Sep. Purif. Technol., 2020, vol. 242, p. 116779.
P. Meshram, and B.D. Pandey: Miner. Process. Extr. Metall. Rev., 2018, vol. 40, pp. 157–93.
B. Li, Z. Ding, Y. Wei, H. Wang, Y. Yang, and M. Barati: Metall. Mater. Trans. B , 2018, vol. 49, pp. 3067–73.
R.G. McDonald and B.I. Whittington: 2008, Hydrometallurgy. vol. 91, pp. 35–55.
C.K. Thubakgale, R.K.K. Mbaya, and K. Kabongo: Int. J. Chem. Mol. Nucl. Mater. Metall. Eng., 2012, vol. 6, pp. 761–5.
U. Soelistijo: Earth Sci. Sci. Publ. Group, 2013, vol. 2, pp. 129–38.
C.R.M. Butt and D. Cluzel: Elements, 2013, vol. 9, pp. 123–8.
Investing News Network (INN): Base Metals Price and Investing Opportunities, Vancouver, 2017.
V. de AlvarengaOliveira, C.G. dos Santos, and E. de AlbuquerqueBrocchi: Metall. Mater. Trans. B , 2019, vol. 50, pp. 1309–21.
U.S. Geological Survey (USGS): Mineral Commodity Summaries 2019, vol. 3, Virginia, Estados Unidos da América, 2019.
A. Briceno and K. Osseo-Asare: Metall. Mater. Trans. B, 1995, vol. 26, pp. 1123–31.
M. Jiang, T. Sun, Z. Liu, J. Kou, N. Liu, and S. Zhang: Int. J. Miner. Process., 2013, vol. 123, pp. 32–8.
N.D.H. Munroe: Metall. Mater. Trans. B, 1997, vol. 28, pp. 995–1000.
H. Basturkcu, N. Acarkan, and E. Gock: Int. J. Miner. Process., 2017, vol. 163, pp. 1–8.
S. Kursunoglu and M. Kaya: Int. J. Miner. Process., 2016, vol. 150, pp. 1–8.
W. Luo, Q. Feng, L. Ou, G. Zhang, and Y. Lu: Hydrometallurgy, 2009, vol. 96, pp. 171–5.
J. A. Johnson, B.C. Cashmore, and R.J. Hockridge: Miner. Eng., 2005, vol. 18, pp. 1297–303.
M.A.R. Önal and Y.A. Topkaya: Hydrometallurgy, 2014, vol. 142, pp. 98–107.
R.G. McDonald and B.I. Whittington: Hydrometallurgy, 2008, vol. 91, pp. 56–69.
J. Luo, G. Li, M. Rao, Z. Peng, Y. Zhang, and T. Jiang: Miner. Eng., 2015, vol. 78, pp. 38–44.
B.K. Loveday: Miner. Eng., 2008, vol. 21, pp. 533–8.
J. Li, K. Bunney, H.R. Watling, and D.J. Robinson: Miner. Eng., 2013, vol. 41, pp. 71–8.
G. Li, T. Shi, M. Rao, T. Jiang, and Y. Zhang: Miner. Eng., 2012, vol. 32, pp. 19–26.
H. Basturkcu and N. Acarkan: Physicochem. Probl. Miner. Process., 2016, vol. 52, pp. 564–74.
X. Guo, D. Li, K.H. Park, Q. Tian, and Z. Wu: Hydrometallurgy, 2009, vol. 99, pp. 144–50.
J. Li, X. Li, Q. Hu, Z. Wang, Y. Zhou, J. Zheng, W. Liu, and L. Li: Hydrometallurgy, 2009, vol. 99, pp. 84–8.
F. O’Connor, W.H. Cheung, and M. Valix: Int. J. Miner. Process., 2006, vol. 80, pp. 88–99.
X.J. Zhai, Q. Wu, Y. Fu, L.Z. Ma, C.L. Fan, and N.J. Li: Trans. Nonferrous Met. Soc. China , 2010, vol. 20, pp. s77–81.
B. Ma, W. Yang, Y. Pei, C. Wang, and B. Jin: Hydrometallurgy, 2017, vol. 169, pp. 411–7.
K.B. Krauskopf: Geochim. Cosmochim. Acta, 1956, vol. 10, pp. 1–26.
R.O. Fournier and J.J. Rowe: The solubility of amorphous silica in water at high temperatures and high pressures|American Mineralogist|GeoScienceWorld, https://pubs.geoscienceworld.org/msa/ammin/article-abstract/62/9-10/1052/104609/The-solubility-of-amorphous-silica-in-water-at?redirectedFrom=fulltext. Accessed 14 August 2020.
Acknowledgments
The authors wish to thank Instituto Tecnológico Vale/Vale S.A, the Brazilian research agency CNPq, and Prof. Dilson Silva dos Santos (COPPE/UFRJ). RN acknowledges financial support from CNPq (Grant 315472/2018-9).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted September 15, 2020; accepted March 3, 2021.
Rights and permissions
About this article
Cite this article
Ribeiro, P.P.M., dos Santos, I.D., Neumann, R. et al. Roasting and Leaching Behavior of Nickel Laterite Ore. Metall Mater Trans B 52, 1739–1754 (2021). https://doi.org/10.1007/s11663-021-02141-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02141-6