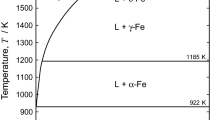
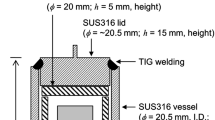
Abstract
Steel containers and instruments are used to hold and transport Mg melts. Thus, quantitative analysis of the dissolution of metallic elements from the steel materials into the liquid Mg is important for controlling the impurities in processes such as Mg alloy production and Ti smelting. When austenitic stainless steels containing Cr and Ni come in contact with a Mg or Mg alloy melt, a large amount of Ni and limited amounts of Fe and Cr dissolve into the melt. In this study, the composition of a Mg-Ni-based melt in equilibrium with SUS316 was investigated to determine the maximum amounts of impurities dissolved from SUS316 into the liquid Mg. Mg-Ni melts of different compositions were held in a closed crucible of SUS316 at 1073 K (800 °C) to 1273 K (1000 °C), and the compositions of the inner wall of the crucible and the Mg-Ni alloy were evaluated after quenching. Subsequently, the relationships between the solubility limit of element i (i: Ni, Fe, and Cr) in liquid Mg with the coexistence of SUS316, \( C_{{{\text{sol}},{i}}}^{*} \) (mass pct), and the temperature, T (K), were determined: \( \log \,(C_{{{\text{sol}},{\text{Ni}}}}^{*} ) = {{ - 1.40 \times 10^{3} }}/{T} + 2.57 \, ( \pm \,0.12), \)\( \log \,(C_{{{\text{sol}},{\text{Fe}}}}^{*} ) = - {{5.20 \times 10^{3} }}/{T} + 3.93 \, ( \pm \,0.16),\;{\text{and}} \)\( \log \,(C_{{{\text{sol}},{\text{Cr}}}}^{*} ) = - {{5.96 \times 10^{3} }}/{T} + 3.80 \, ( \pm \,0.05). \) These solubility limits were compared with those estimated based on the available thermodynamic data for the SUS316 and Mg-i binary systems. The validity of the obtained data and the reliability of the previously reported thermodynamic data were also discussed.















Similar content being viewed by others
References
1.O. Kubaschewski: Iron-Binary Phase Diagrams, Springer, New York, NY, 1982, pp. 59-60.
A.A. Nayeb-Hashemi, J.B. Clark, and L.J. Swartzendruber: Bull. Alloy Phase Diagrams, 1985, vol. 6, pp. 235-38.
Handbook of Extractive Metallurgy, F. Habashi, ed., VCH Verlagsgesellschaft mbH, Weinheim, 1997.
4.H. Kusamichi, J. Iseki, A. Moriya, A. Kanai, T. Nishimura, H. Kanayama, and T. Kusamichi: Titanium Industry in Japan and Its New Technologies, AGNE Gijutsu Center, Tokyo, 1996 (in Japanese).
5.J.D. Hanawalt, C.E. Nelson, and J.A. Peloubet: Trans. AIME, 1942, vol. 147, pp. 273-99.
Magnesium Technology: Metallurgy, Design Data, Applications, H.E. Friedrich and B.L. Mordike, eds., Springer, Berlin, 2006.
H. Okamoto: J. Phase Equilib., 2000, vol. 21, p. 209.
8.J. Buha: Mater. Sci. Eng. A, 2008, vol. 492, pp. 293-99.
A.A. Nayeb-Hashemi and J.B. Clark: Bull. Alloy Phase Diagrams, 1985, vol. 6 (3), pp. 238-44.
FactSage Thermochemical Software (version 7.3) and Databases (FTlite and FSstel), www.factsage.com.
K. Sakakibara: J. JFS, 2014, vol. 86, pp. 639-51 (in Japanese).
K. Kaitoh, T. Motegi, and E. Satoh: J. Jpn. Inst. Light Met., 1995, vol. 45, pp. 708-12 (in Japanese).
13.C. Scharf and A. Ditze: Adv. Eng. Mater., 2007, vol. 9 (7), pp. 566-71.
C.K. Tang, M.-A.V. Ende, and I.-H. Jung: in Magnesium Technology 2012, Proc. TMS 2012 Annual Meeting and Exhibition (TMS2012), Springer, Cham, 2012, pp. 261–64.
M. Sonoda, Y. Takahashi, H. Era, and N. Shinozaki: J. JFS, 2016, vol. 88, pp. 663-67 (in Japanese).
16.Y. Taninouchi, K. Nose, and T.H. Okabe: Metall. Mater. Trans. B, 2018, vol. 49B, pp. 3432-43.
T. Chen, X. Xiong, Y. Yuan, A. Tang, D. Li, A. Atrens, and F. Pan: Adv. Eng. Mater., 2020, vol. 22 (11), no. 2000338.
Japanese Industrial Standards: JIS G 4305, 2012.
Japanese Industrial Standards: JIS G 3459, 2016.
T. Koyama, M. Kanno, and M. Yamawaki: Mass Spectrosc., 1987, vol. 35 (2), pp. 56-62.
21.V. Venugopal, S.G. Kulkarni, C.S. Subbanna, and D.D. Sood: J. Alloys Compd., 1995, vol. 218, pp. 95-100.
22.A.M. Azad, O.M. Sreedharan, and J.B. Gnanamoorthy: J. Nucl. Mater., 1987, vol. 144, pp. 94-104.
23.A.M. Azad, O.M. Sreedharan, and J.B. Gnanamoorthy: J. Nucl. Mater., 1989, vol. 167, pp. 82-88.
24.N.P. Nawada and O.M. Sreedharan: J. Nucl. Mater., 1999, vol. 273, pp. 37-51.
The SGTE Unary Database, version 5.0, http://www.crct.polymtl.ca/sgte/index.php?free=1.
COST 507, Thermodynamical Database for Light Metal Alloys, I. Ansara, A.T. Dinstale, and M.H. Rand, eds., European Communities, Luxembourg, 1998, vol. 2, pp. 143–44.
Acknowledgments
The authors are grateful to Dr. Katsuhiro Nose, University of Tokyo (currently with TANIOBIS GmbH); Messrs. Kazuhiro Taki, Masanori Yamaguchi, Yosuke Inoue, and Meiji Watanabe, Toho Titanium Co., Ltd.; and Professor Hiroyuki Matsuura, University of Tokyo, for valuable comments and suggestions. The authors also thank Messrs. Eiji Shirane and Yoji Iwai, Toho Titanium Co., Ltd., for their assistance in carrying out the composition analysis with ICP-AES. This article is based on results obtained from a project, JPNP14014, commissioned by the New Energy and Industrial Technology Development Organization (NEDO). This research was partly supported by the Japan Society for the Promotion of Science (JSPS) through a Grant-in-Aid for Scientific Research (S) (KAKENHI Grant No. 19H05623).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted June 30, 2020; accepted October 31, 2020.
Rights and permissions
About this article
Cite this article
Taninouchi, Yk., Okabe, T.H. Solubilities of Nickel, Iron, and Chromium in Liquid Magnesium in the Presence of Austenitic Stainless Steel. Metall Mater Trans B 52, 611–624 (2021). https://doi.org/10.1007/s11663-020-02025-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-020-02025-1