Abstract
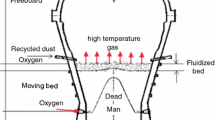
A devolatilization study of two lump coals used in China COREX3000 was carried out in a self-developed thermo-gravimetry at four temperature conditions [1173 K, 1273 K, 1373 K, and 1473 K (900 °C, 1000 °C, 1100 °C, and 1200 °C)] under N2. This study reveals that the working temperature has a strong impact on the devolatilization rate of the lump coal: the reaction rate increases with the increasing temperature. However, the temperature has little influence on the maximum mass loss. The conversion rate curve shows that the reaction rate of HY lump coal is higher than KG lump coal. The lump coals were analyzed by XRD, FTIR, and optical microscopy to explore the correlation between devolatilization rate and properties of lump coal. The results show that the higher reaction rate of HY lump coal attributes to its more active maceral components, less aromaticity and orientation degree of the crystallite, and more oxygenated functional groups. The random nucleation and nuclei growth model (RNGM), volume model (VM), and unreacted shrinking core model (URCM) were employed to describe the reaction behavior of lump coal. It was concluded from kinetics analysis that RNGM model was the best model for describing the devolatilization of lump coals. The apparent activation energies of isothermal devolatilization of HY lump coal and KG lump coal are 42.35 and 45.83 kJ/mol, respectively. This study has implications for the characteristics and mechanism modeling of devolatilization of lump coal in COREX gasifier.














Similar content being viewed by others
Abbreviations
- XRD:
-
X-ray diffraction
- FTIR:
-
Fourier transform infrared spectroscopy
- RNGM:
-
Random nucleation and nuclei growth model
- VM:
-
Volume model
- URCM:
-
Unreacted shrinking core model
- \( \frac{{{\text{d}}\alpha }}{{{\text{d}}t}} \) :
-
Reaction conversion rate (s−1)
- k(T):
-
The reaction rate constant
- f(α):
-
The devolatilization mechanism function
- t :
-
Devolatilization time (s)
- α :
-
Devolatilization conversion
- A :
-
The pre-exponential factor (s−1)
- E :
-
The activation energy (kJ/mol)
- R:
-
The universal gas constant (kJ/(mol K))
- m i :
-
The initial weight of lump coal (g)
- m t :
-
The instant weight of the sample during experiment (g)
- m ∞ :
-
The sample weight after experiment (g)
- k RNGM :
-
The reaction rate constant of random nucleation and nuclei growth model
- k VM :
-
The reaction rate constant of volume model
- k URCM :
-
The reaction rate constant of unreacted shrinking core model
- \( \left( {\frac{{{\text{d}}\alpha }}{{{\text{d}}t}}} \right)_{\exp ,i} \) :
-
Reaction conversion rate based on the experiment data
- \( \left( {\frac{{{\text{d}}\alpha }}{{{\text{d}}t}}} \right)_{{{\text{calc}},i}} \) :
-
Reaction conversion rate based on the experiment data
- i :
-
The reaction temperature (K)
- DEV(α):
-
Relative error (pct)
- α exp,i :
-
Experiment devolatilization conversion data
- α calc,i :
-
Calculated devolatilization conversion data
- max(α)exp :
-
The maximum conversion of experiment
- N :
-
The number of experiment dada
- K1 :
-
Correction coefficient
- A 002 :
-
The area of (002) band
- A γ :
-
The area of γ band
- B 002 :
-
The half width of (002) band (deg)
- ϕ 002 :
-
The diffraction angle of (002) band (deg)
- λ :
-
The wavelength of incident rays, nm
- θ 100 :
-
The diffraction angle of (100) band (deg)
- β 100 :
-
THE half width of (100) band (deg)
References
S. Pal and A. K. Lahiri: Metallurgical and Materials Transactions B., 34 (2003),103–14.
S. Pal and A. K. Lahiri: Metallurgical and Materials Transactions B., 34(2003),115–19.
3. X. Liu, G. Pan, G. Wang and Z. Wen: Energy & Fuels, 25(2011),5729–35.
4. K. Du, S.Wu, Z. Zhang, F. Chang and X. Liu: ISIJ International, 54(2014), 2737–45.
5. Y. Guo, W. Xu, J. Zhu and J. Zhang: Metallurgical and Materials Transactions B., 44(2013), 1078–85.
6. P. P. Kumar, D. Gupta, T. K. Naha and S. S. Gupta: Ironmaking & Steelmaking., 33(2006), 293–98.
7. Y. L. Guo, W. Xu, J. Zhu and J. Zhang: Ironmaking & Steelmaking., 40(2013), 545–50.
8. P. P. Kumar, S. C. Barman, B. M. Reddy and V. R. Sekhar: Ironmaking & Steelmaking., 36(2009), 87–90.
9. B. Kim, S. Gupta, S. Lee, S. Kim and V. Sahajwalla: Energy & Fuels., 22(2008), 514–22.
10. M. Minkina, F. L. G. Oliveira and V. Zymla: Fuel Processing Technology., 91(2010), 476–85.
11. R. Sahoo and D. Roach: Powder Technology., 152(2005), 1–8.
12. R. Sahoo and D. Roach: Chemical Engineering and Processing: Process Intensification., 44(2005), 797–804.
13. Q. P. Campbell, J. R. Bunt and F. de Waal: Journal of Analytical and Applied Pyrolysis., 89(2010), 271–77.
S. Zhang, H. Peng, X. Zhang, W. Liu, L. Wen and G. Qiu: Fuel Process. Technol., 129(2015), 174–82.
M. Knepper, A. Babich, and D. Senk: Proc. 6th Int. Congress on the Science and Technology of Ironmaking - ICSTI, Rio de Janeiro, Brazil, 2012, pp. 811–21.
16. S. Zhang, F. Zhu, C. Bai, L. Wen and H. Peng: Ironmaking & Steelmaking., 41(2014), 219–28.
17. L. T. Vlaev, I. G. Markovska and L. A. Lyubchev: Thermochimica Acta.; 406(2003):1–7.
L. Wang, J. Cai, M. Li and H. Sun: Journal of Northeastern University, 31:(2010):550–54.
19. S. Kasaoka, Y. Sakata and C. Tong: Int. Chem. Eng., 25(1984), 160–67.
20. J.Y. Shang and E.W. Eduard: Fuel, 63(1984), 1604–09.
21. C. Xu, S. Hu, J. Xiang, H. Yang, L. Sun, S. Su, B. Wang, Q. Chen, L. He: Bioresource Technology, 171(2014), 253–59.
22. J. Szekely, J.W. Evans: Chem. Eng. Sci., 25 (1970), 1091–107.
R. Xu, J. Zhang, G. Wang, H. Zuo, P. Li, H. Wang, H. Lin and S. Liu: J. Thermal Anal. Calorim., 123(2016), 773–83.
H. Xiao, J. Zhang, F. Jia and Q. Pang: Iron and Steel, 49(2014), 29-33. (in Chinese)
25. B. Feng, S. K. Bhatia and J. C. Barry: Carbon., 40(2002), 481–96.
26. G. Wang, J. Zhang, X. Hou, J. Shao and W. Geng: Bioresource Technol.,177 (2015):66–73.
27. Y. Sekine, K. Ishikawa, E. Kikuchi, M. Matsukata and A. Akimoto: Fuel,85(2006): 122–26.
28. K. Xie. Coal structure and its reactivity[M], first edition, science press,Beijing, China, 2012,pp. 210–12
29. J. Yu, J. A. Lucas and T. F. Wall: Progress in Energy and Combustion Science., 33(2007), 135–170.
30. Y. Bai, Y. Wang, S. Zhu, L. Yan, F. Li, K. Xie: Fuel, 126 (2014), 1–7.
31. L. Zhang, W. Liu, D. Men: International Journal of Mining Science and Technology, 24(2014), 93–98.
32. R. Walker, M. Mastalerz. : International Journal of Coal Geology, 58(2004), 181–91.
33. W. Xie: Coal chemical and coal quality analysis, first edition, Metallurgical industry press, Beijing, China, 2012, pp. 22–25.
34. K. Xie. Coal structure and its reactivity[M], first edition, science press, Beijing, China, 2012,pp.68–70
35. L. Lu, V. Sahajwalla, C. Kong and D. Harris: Carbon., 39(2001), 1821–33.
36. O. O. Sonibare, T. Haeger and S. F. Foley: Energy, 35(2010), 5347–53.
37. S. Gupta, V. Sahajwalla, P. Chaubal and T. Youmans: Metallurgical and Materials Transactions B., 36(2005), 385–94.
38. M. Kawakami, H. Kanba, K. Sato, T. Takenaka, S. Gupta and R. Chandratilleke, V. Sahajwalla: ISIJ International., 46(2006), 1165–70.
39. P.R. Solomon, D.G. Hamblen, R.M. Carangelo M.A. Serio and G.V. Deshpande: Energy &Fuels, 2(1988),405–22.
Acknowledgments
The authors acknowledge the financial support for this work provided by the National Basic Research Program of China (973 Program) (No. 2012CB720401), National Natural Science Foundation of China (No. 51574023), and National Key technology R&D Program of China (No. 2011BAC01B02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted October 14, 2015.
Rights and permissions
About this article
Cite this article
Xu, R., Zhang, J., Wang, G. et al. Devolatilization Characteristics and Kinetic Analysis of Lump Coal from China COREX3000 Under High Temperature. Metall Mater Trans B 47, 2535–2548 (2016). https://doi.org/10.1007/s11663-016-0708-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0708-8