Abstract
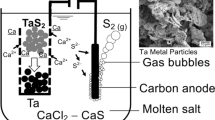
A process that uses the solid-oxide–oxygen-ion conducting membrane has been investigated to produce tantalum directly from solid Ta2O5 in molten CaCl2 or a molten mixture of 55.5MgF2-44.5CaF2 (in wt pct). The sintered porous Ta2O5 pellet was employed as the cathode, while the liquid copper alloy, saturated with graphite powder and encased in a one-end-closed yttria-stabilized-zirconia (YSZ) tube, acted as the anode. The electrolysis potential in this method is higher than that of the Fray–Farthing–Chen Cambridge process because the YSZ membrane tube blocks the melts to electrolyze, and only Ta2O5 is will be electrolyzed. The microstructures of reduced pellets and a cyclic voltammogram of solid Ta2O5 in molten CaCl2 were analyzed. In addition, the influence of particle size and porosity of the cathode pellets on metal-oxide-electrolyte, three-phase interlines was also discussed. The results demonstrate that the sintering temperature of cathode pellets and electrolytic temperature play important roles in the electrochemical process. Furthermore, this process can be used to produce Ta metal efficiently without the expensive cost of pre-electrolysis and generation of harmful by-products.










Similar content being viewed by others
Abbreviations
- \( \Theta_{\text{a}} \) :
-
Length of the ideal 3PIs (m)
- A :
-
Cross-section area of the pellet (m2)
- δ :
-
Porosity of the pellet (percent)
- γ :
-
Average particle size of the pellet (m)
- L :
-
Half of pellet thickness (m)
- x :
-
Position along the thickness direction of the pellet, 0 ≤ x ≤ L (m)
- t :
-
Electrolysis time, t ≤ 2 hours (minutes)
- n :
-
Shape factor of the pellet, n ≥ 2 (−)
References
1. F. Cardarelli, P. Taxil, A. Savall, C. Comninellis, G. Manoli, and O.Leclerc: J. Appl. Electrochem., 1998, vol. 28, pp. 245-54
2. L. Massot, P. Chamelot, P. Palau, and P. Taxil: Electrochim. Acta, 2005, vol. 50, pp. 5408-13
3. M. Baba, Y. Ono, and R.O. Suzuki: J. Phys. Chem. Solids, 2005, vol. 66, pp. 466-70
4. M.A. Hunter: J. Metals., 1953, vol. 5, pp. 130-2
5. Π. Park, T.H. Okabe, and Y. Waseda: J. Alloys Comp., 1998, vol. 280, pp. 265-72
6. H. Niiyama, Y. Tajima, F. Tsukihashi, and N. Sano: J. Alloys Comp., 1991, vol. 169, pp. 209-16
7. A. Krishnan, X.G. Lu, and U.B. Pal: Scand. J. Metall., 2005, vol. 34, pp. 1-9
8. G.Z. Chen, D.J. Fray, and T.W. Farthing: Nature, 2000, vol. 407, pp. 361-4
9. G.Z. Chen, D.J. Fray, and J. Electro: Chem. Soc., 2002, vol. 149, pp. 455-67
10. G.Z. Chen, E. Gordo, and D.J. Fray: Metall. Mater. Trans. B, 2004, vol. 35, pp. 223-33
11. X. Van and D. Fray: Metall. Mater. Trans. B, 2002, vol. 33, pp. 685-93
12. D.T.L. Alexander, C. Schwandt, and D.J. Fray: Acta Mater., 2006, vol. 54, pp. 2933-44
13. R. Enmei, T. Kikuchi, and R.O. Suzuki: Electrochimica Acta, 2013, vol. 100, pp. 257-60
14. X. Jin, P. Gao, D. Wang, X. Hu, and G.Z. Chen: Angew. Chem. Int. Edit., 2004, vol. 43, pp. 733-6
15. U.B. Pal, D.E. Woolley, and G.B. Kenney: J. Metals, 2001, vol. 10, pp. 32-6
16. U.B. Pal, D.E. Woolley, A. Krishnan, T. Keenan, C.P. Manning, and G.B. Kenney: Magnesium Technology, TMS, Warrendale, PA, 2002, p. 19-24
17. C.Y. Chen, X.G. Lu, and Q. Li et al.: Chin. J. Rare Metals. (in Chinese), 2007, vol. 31, pp. 306-10
18. U.B. Pal, A. Krishnan, and X.G. Lu: JOM, 2004, vol. 56, pp. 245-53
19. C.Y. Chen and X.G. Lu: Acta Metall. Sin. (in Chinese), 2008, vol. 44, pp. 163-7
20. A. Krishnan: Solid Oxide Membrane Process for The Direct Reduction of Magnesium from Magnesium Oxide, Boston University, Boston, MA, 2006, pp. 25–49
21. H.W. Cheng, X.G. Lu, Q. Li, J.M. Liu, W.Z. Ding, and G.Z. Zhou: Acta Metall. Sin. (in Chinese), 2006, vol. 42, pp. 500-05
22. R.O. Suzuki, M. Baba, Y. Ono, and K. Yamamoto: J. Alloys Comp., 2005, vol. 389, pp. 310-6
24. E. Gordo, G.Z. Chen, and D.J. Fray: Electrochim. Acta, 2004, vol. 49, pp. 2195-2208
25. X.F. Hu and Q. Xu: Acta Metall. Sin. (in Chinese), 2006, vol. 42, pp. 285-9
26. X.Z. Xu, M. Zhu, and J.M. Yang: Eng. Sci. (in Chinese), 2005, vol. 7, pp. 36-41
27. T. Wu, X.B. Jin, W. Xiao, X.H. Hu, D.H. Wang, and G.Z. Chen: Chem. Mater., 2007, vol. 19, pp. 153-60
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Nos. 51264006, 51474079, 51574095), the Basic Research Program of Guizhou Provincial Education Department (No. 20120002), and the Industrial Projects Guiyang Municipal Science and Technology Bureau ([2012103]69, [2012205]64), KY(2015)334.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted April 24, 2015.
Rights and permissions
About this article
Cite this article
Chen, C., Yang, X., Li, J. et al. Direct Electrolytic Reduction of Solid Ta2O5 to Ta with SOM Process. Metall Mater Trans B 47, 1727–1735 (2016). https://doi.org/10.1007/s11663-016-0633-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0633-x