Abstract
Platinum (Pt) is typically recovered by employing dissolution processes in aqueous solutions; however, these processes require a long processing time and considerable quantities of acids with strong oxidants owing to the high chemical stability of Pt. In order to develop an efficient dissolution process, we studied chlorination treatments for Pt prior to dissolution. Chlorination was carried out at 673 K to 873 K (400 °C to 600 °C) using copper(II) chloride (CuCl2) as a chlorine source. While pure Pt was insoluble in hydrochloric acid (HCl(aq)), the entire Pt component of the treated sample dissolved in HCl(aq) under certain conditions. Therefore, the proposed method can be used as a new, environmental friendly Pt recovery process.
Similar content being viewed by others
Introduction
Platinum (Pt) is characterized by high heat resistance, high corrosion resistance, and unique catalytic properties, and therefore, it is widely used in many product applications, e.g., for manufacturing autocatalysts and electronic parts. In the coming years, it is expected that the demand for Pt will increase because of stricter environmental regulations and because of its potential application in fuel cells.[1,2] Therefore, Pt is considered an important industrial element.
The platinum group metals (PGMs) including Pt are one of the rarest elements found on Earth; its concentration in natural ores is quite low, being approximately 5 ppm even in the highest grade ore.[2–4] Worldwide platinum reserves are quite disproportionate, with South Africa and Russia.[2–12] The global demand for Pt in 2010 was 220 tons, of which approximately 170 tons was supplied from mines and 50 tons was supplied from recycled materials.[5] Since PGMs are generally associated with nickel-copper sulfide in magmatic rocks, PGMs are produced along with nickel and copper.[3] However, in South Africa, PGMs are produced as the primary products from the viewpoint of the economy.[3] The whole production process for PGMs in South Africa is classified into three individual processes: (1) on-mine, (2) smelting, and (3) treatment and refining. The cost involved in on-mine processes, such as mining, grinding, and mineral dressing, accounts for 80 pct of the whole production process cost.[4] To summarize, recycling of PGMs is favorable in terms of cost reduction in the on-mine process, decreasing environmental burden, and mitigating the potential risks of supply related to the disproportionate distribution of the reserves.
Pt is generally recovered by pyrometallurgical and hydrometallurgical processes.[3,9,10,13–16] In pyrometallurgical processes, PGMs are enriched by utilizing a liquid metal such as copper (Cu) as a collector at high temperatures.[13] Subsequently, PGMs are dissolved by acid or alkaline solutions in hydrometallurgical processes, followed by separation using solvent extraction,[14] ion exchange,[14–16] etc. While these processes possess high efficiency and speed, they consume considerable energy and require large-scale equipment. In contrast, in the viewpoints of less energy and less bulky equipment, another type of methods exists, in which the dissolution of PGMs directly from the scraps is carried out in hydrometallurgical processes.
The problems with the recycling process for Pt are low efficiency, requirement of large quantities of acids, and long processing times for the dissolution of Pt due to its high chemical stability. As a result, strong oxidants such as aqua regia are necessary for its dissolution. However, the dissolution of Pt using an oxidant results in the emission of toxic gases; for example, nitrocyl chloride (NOCl) and chlorine (Cl2) gases are formed when aqua regia is used. Although direct dissolution of PGMs from scraps, such as treatment with hydrochloric acid (HCl(aq)) and Cl2 gas, is proposed,[17,18] the use of strong oxidants is indispensable. Therefore, if the dissolution efficiency of Pt is improved by pretreatment in the pyrometallurgical process, then it is expected to shorten the processing and to establish an environmentally friendly process without an evolution of toxic gases in the dissolution process.
With these backgrounds, our group investigated an effective dissolution method for Pt that utilized a metal vapor alloying treatment.[19–22] When this treatment was carried out using the vapor of reactive metals (R) such as magnesium (Mg) to form Mg-Pt compounds, the dissolution efficiency of Pt improved in aqua regia (a mixture of HCl [35 pct, 30 mL] and nitric acid [HNO3, 70 pct, 10 mL]). In the dissolution experiments, only 14 pct of pure Pt dissolved in 4 hours, whereas the Pt component from the Mg-Pt compounds completely dissolved in 1 hour.[19] This finding could be explained by the increase in the Pt surface area because of the prior dissolution of Mg in the Mg-Pt compounds. However, both pure Pt and the Mg-Pt compounds were insoluble in HCl(aq). These results indicated that even with the alloying treatment, strong oxidants were required for dissolving Pt. Herein, we report the efficient dissolution of Pt in HCl(aq) without using any oxidants as part of a series of pretreatment methods.
Thermodynamic Analysis
First, we investigated the dissolution conditions for Pt by thermodynamic analysis. The stability of Pt species in chloride-ion-containing solutions can be discussed on the basis of the potential-pCl diagram.[23–25] Figure 1 shows the potential-pCl diagram for the Pt-Cl-H2O system at 298 K (25 °C) and pH = 0. The ordinate is the electrochemical potential E with respect to the standard redox potential of a standard hydrogen electrode (SHE). The abscissa is the reciprocal of the common logarithm of chloride ion activity pCl, which is defined as \({\text{pCl}} = -{\text{log}}\;{a_{{\text{Cl}}^{-}}}.\)
The diagram shows that under the high chemical potential of chloride ions, i.e., in the low pCl region, the complex ions PtCl6 2− and PtCl4 2− are more stable than Pt chlorides and Pt oxides. It has been reported that all Pt chlorides are soluble in HCl(aq).[26,27] Therefore, it is expected that chlorinated (or oxidized) Pt can be dissolved in HCl(aq) without using strong oxidants.
Next, the optimum chlorination conditions for Pt were investigated. According to the literature, PtCl2 is produced by heating Pt metal in Cl2 gas at 923 K (650 °C) followed by cooling to 773 K (500 °C).[28] The stable temperature ranges of Pt chlorides in the presence of Cl2 gas are reported to be lower than 655 K (382 °C) for platinum tetrachloride (PtCl4, red brown), 655 K to 723 K (382 °C to 450 °C) for platinum trichloride (PtCl3, dark green), and 708 K to 788 K (435 °C to 515 °C) for platinum dichloride (PtCl2, green-brown).[27] Therefore, the chlorination temperatures selected in this study were 673 K, 773 K, and 873 K (400 °C, 500 °C, and 600 °C).
Figure 2 shows the Ellingham diagram of some chlorides, as well as the standard Gibbs energy of reaction between copper(I) chloride (CuCl) and copper(II) chloride (CuCl2).[29] In order to chlorinate Pt, the standard Gibbs energy of the chlorine source (oxidizer) should be greater than that for the formation of PtCl2. In the temperature range between 673 K and 873 K (400 °C and 600 °C), Figure 2 indicates that, thermodynamically, Cl2 gas or CuCl2 (which decomposes into CuCl and Cl2) can be used as the chlorine source (oxidizer). From the viewpoint of safety and ease of handling, CuCl2 was selected in this study.
Ellingham diagram of some chloride salts, and the Standard Gibbs energy of reaction discussed in this study. Solid and broken lines indicate the original reference data and extrapolated data, respectively[29]
Experimental
Chlorination Methods
The chlorination treatments of Pt using CuCl2 were conducted by two methods: a gas-phase reaction and a reaction by physical mixture. Figure 3 shows the schematic illustrations and photographs of the experimental apparatus for chlorination using CuCl2. In the gas-phase reaction in Figure 3(a), pure Pt and CuCl2 powders were placed in separate quartz crucibles (inner diameter [ID]: 27 mm, height: 21 mm). When the chlorination treatment is performed, Cl2 gas generated from CuCl2 is supplied as the chlorine source to react with Pt in the vapor phase.
In the reaction by physical mixture shown in Figure 3(b), a mixture of pure Pt and CuCl2 powders was placed in a quartz crucible. When the chlorination treatment is performed, Pt can be chlorinated by both Cl2 gas and CuCl2.
The masses of pure Pt were approximately 0.4 g. The molar amount of CuCl2 was required to be four times that of Pt metal in order to ensure sufficient chlorine supply. In both methods, the crucible(s) was/were installed in a quartz tube (ID: 42 mm, length: 450 mm) sealed with a silicone stopper. Glass wool (Fine 10; Tosoh Corp., Tokyo, Japan) was also installed to reduce the dissipation of the powdered and vaporized samples. Before chlorination, the quartz tube was evacuated using a rotary pump, and then the vacuum line was closed. Then, chlorination treatment was performed by heating the quartz tube in a horizontal furnace maintained at 673 K, 773 K, and 873 K (400 °C, 500 °C, and 600 °C) for 3 hours. The internal pressure of the tube was measured using a pressure gauge. After chlorination, the quartz tube was removed from the furnace and cooled at room temperature.
In case of the chlorination for Mg-Pt compounds, Mg-Pt compounds were prepared based on previous research[19] and treated in the same way as pure Pt.
Dissolution of Chlorinated Samples
The dissolution experiment was carried out by immersing 0.05 to 0.1 g of the chlorinated sample for 15 minutes in 10 M HCl aq. (45 mL) maintained at 353 K (80 °C) by a temperature-controlled bath. The ratio of dissolved Pt to the Pt component in the sample was determined. After the dissolution experiments, the residues in the leaching solution were filtrated using a filter paper (5C, pore size: 0.03 μm, diameter: 55 mm; Advantec Co., Ltd., Tokyo, Japan). The obtained solution was diluted to 100 mL with deionized water.
Analysis
The crystalline phases in the sample were identified by X-ray diffraction analysis (XRD; RINT 2000; Rigaku Co., Ltd., Tokyo, Japan). In the XRD analysis, the chlorinated samples were covered using parafilm to avoid reaction with atmospheric moisture. The solid samples were observed using scanning electron microscopy (SEM; JSM-5600LV; JEOL Co., Ltd., Tokyo, Japan). Qualitative chemical analyses of the samples were carried out using X-ray fluorescence spectroscopy (XRF; JSX-3210; JEOL Co., Ltd.). A quantitative chemical analysis of the solution was carried out using inductively coupled plasma-atomic emission spectrometry (ICP-AES) (SPS 4000; Seiko Instruments Inc., Chiba, Japan). The fractions of dissolved Pt, \( R^{\prime}_{\text{Pt}} \) (pct), in the dissolution experiments were calculated as follows:
where \( C^{\prime}_{\text{Pt}} \) is the concentration of Pt in the 100-mL solution in ppm and w l,Pt is the amount of Pt in grams in the feed sample, whose Pt content was determined by ICP-AES.
The masses and compositions of the samples after the chlorination treatments as determined by ICP-AES and detected by XRF are summarized in Table I. XRF analysis was used to confirm chlorination of Pt. For the reaction by physical mixture and the samples from the Mg-Pt compounds, XRF analysis was not conducted because it is difficult to identify chlorination of Pt from MgCl2 and CuCl x (x = 1, 2). The fractions of dissolved Pt, \( R^{\prime}_{\text{Pt}} \), are summarized in Table II.
Results And Discussion
Effect of the Chlorination Temperatures
The gas-phase chlorination reactions were carried out at 673 K, 773 K, and 873 K (400 °C, 500 °C, and 600 °C) in order to investigate the effect of temperature. When the quartz tube was heated in the furnace, the internal pressure of the tube increased at the outset and became constant in 30 minutes. The pressures at 673 K, 773 K, and 873 K (400 °C, 500 °C, and 600 °C) were approximately 0.1 atm, 0.3 atm, and 0.4 atm, respectively. These behaviors are explained by the generation of Cl2 gas from CuCl2 according to the following reaction[29]:
To synthesize PtCl2, the partial pressure of Cl2 needs to be higher than approximately 10−6 atm, 10−5 atm, and 10−4 atm at 673 K, 773 K, and 873 K (400 °C, 500 °C, and 600 °C), respectively.[29] Therefore, it is suggested that sufficient chlorine was provided to the Pt powder during heating. After chlorination, the pure Pt powder turned gray at 673 K and 873 K (400 °C and 600 °C), and it turned dark green at 773 K (500 °C).
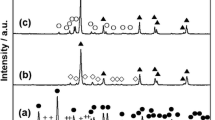
Figure 4 shows the dependence of the dissolved fractions on the chlorination temperature of pure Pt for the gas-phase reactions. While pure Pt before chlorination is insoluble in HCl(aq), the ratio of dissolved Pt for the samples chlorinated at 773 K (500 °C) reaches 0.7 pct, which is the highest among the temperatures investigated. Figure 5 shows the XRD patterns of the samples obtained by chlorination at 773 K (500 °C). Although a clear peak attributed to PtCl2 at around 35 deg is not observed in Figure 5(a), the XRF analysis detected 3.6 to 9.0 mass pct Cl only for the samples at 773 K (500 °C), as shown in Table I. These results suggest that the dissolution of Pt for the samples at 773 K (500 °C) is efficient because Pt chlorides were produced by chlorination treatment. Using XRF, the Cu component can be detected from the amount of CuCl2 evaporated and transported to the surface of Pt samples. Since the Cu component was small, chlorination was considered to be carried out by Cl2 gas.
Effect of Chlorination Methods
The dependence of the dissolved fractions on the two chlorination methods, the gas-phase reaction and the reaction by physical mixture, at 773 K (500 °C) is shown in Figure 6. The ratio of dissolved Pt is 30 times higher in the reactions by physical mixture than in the gas-phase reactions, which proves that chlorination by the former method leads to more efficient dissolution of Pt. The difference in the dissolution rates can be explained by the driving force for chlorination. In the case where Pt is chlorinated directly by CuCl2 in the reactions by physical mixture (Reaction [2]), the chlorine chemical potential, \( p_{{{\text{Cl}}_{2} }}, \) participating in the chlorination is virtually given as 18 atm at 773 K (500 °C) from the standard Gibbs energy of Reaction [3] as shown in Figure 7.[29] On the other hand, when Pt is chlorinated in the gas-phase reactions by Cl2 gas generated by the decomposition of CuCl2 (Reaction [1]), the value of \( p_{{{\text{Cl}}_{ 2} }} \) is at most 1 atm because the internal pressure of the reaction apparatus used cannot exceed atmospheric pressure. Therefore, in addition to \( p_{{{\text{Cl}}_{ 2} }}, \) the driving force for chlorination in the reactions by physical mixture is higher than that in the gas-phase reactions.
Ratio of dissolved Pt in the dissolution experiments in 45 mL of 10 M HCl(aq) at 353 K (80 °C) for 15 min. Specimens were the samples obtained from pure Pt and the Mg-Pt compounds in the gas-phase reactions and in the reactions by physical mixture using CuCl2 at 773 K (500 °C) for 3 h. ↓: No dissolution (did not exceed 0.1 pct)
Chemical potential diagram of the Pt-Cu-Cl system at 773 K (500 °C)[29]
Effect of Alloying Treatment for Pt
Based on the optimum chlorination conditions determined above (773 K [500 °C], physical mixture), the chlorination treatment was also carried out for the Mg-Pt compounds (22 mass pct Mg and 78 mass pct Pt). After chlorination, pure Pt powder turned black, and the Mg-Pt compounds turned dark green.
In the XRD pattern for the sample obtained from pure Pt (Figure 5(b)), in addition to the peaks for pure Pt and CuCl, a peak attributed to PtCl2 is observed at around 35 deg. For the sample obtained from the Mg-Pt compounds (Figure 5(c)), peaks for MgCl2 and CuCl were observed. Mg was chlorinated because Mg has a stronger affinity for chlorine than Pt does, as shown in Figure 2. Because a peak attributed to PtCl2 at around 35 deg overlaps with MgCl2, it is difficult to identify the generation of Pt chlorides. Here, it should be noted that peaks of metallic Pt are not found. This result clearly confirms that Pt chlorides were produced effectively from this sample. The comparison of the dissolved fractions between pure Pt and the Mg-Pt compounds in the reactions by physical mixture at 773 K (500 °C) is also shown in Figure 6. It is found that the dissolution efficiency of Pt by HCl(aq) is significantly improved by the alloying treatment with Mg. This improvement can be attributed to the increase in the surface area of Pt because of the prior chlorination and dissolution of Mg in the Mg-Pt compounds as well as the dissolution in aqua regia.[19] In particular, it is noteworthy that all the Pt in the Mg-Pt compounds is dissolved by HCl(aq) after the chlorination treatments in the reaction by physical mixture at 773 K (500 °C). The observed fractions exceed 100 pct probably due to the measurement accuracy of ICP-AES.
Through a series of fundamental experiments, we revealed that Pt can be completely dissolved in HCl(aq) alone without using solutions containing any strong oxidants. The processing time for the dissolution of Pt is greatly shortened by the improved dissolution efficiency. Based on these results, we propose a new, environmentally friendly process that enables the efficient and high-speed recovery of Pt. The flowchart of the proposed Pt recovery process is shown in Figure 8. After the alloying treatment, chlorination treatment of Pt is performed as a pretreatment for dissolution. The chlorinated Pt is dissolved in HCl(aq) without oxidants. Figure 9 shows the change in \( p_{{{\text{Cl}}_{ 2} }} \) with processing time when Pt is dissolved in chloride-ion-containing acids such as aqua regia and HCl(aq). In the conventional process, \( p_{{{\text{Cl}}_{ 2} }} \) increases during the dissolution process. Typically, long processing times and considerable quantities of acids with strong oxidants are required for the dissolution of Pt. In the new process, while \( p_{{{\text{Cl}}_{ 2} }} \) decreases by the synthesis of R-Pt compounds, it significantly increases during the chlorination treatment by forming Pt chlorides (PtCl x ). Because chlorinated Pt already exists in an oxidized valence state, Pt can be dissolved efficiently in acids even without strong oxidants. According to Figure 1, oxidation treatment might be promising in addition to chlorination treatment. This process has several advantages such as short processing time, low energy cost, and low environmental burden. It is also economical because quick recoveries can respond to fluctuations in the market prices of Pt.
Conclusions
In order to develop an efficient dissolution process for the recovery of Pt, we studied chlorination treatments for Pt prior to dissolution. Chlorination was carried out at 673 K to 873 K (400 °C to 600 °C) using copper(II) chloride (CuCl2) as a chlorine source. While pure Pt was insoluble in hydrochloric acid (HCl(aq)) owing to the high chemical stability, the entire Pt component of the treated sample dissolved in HCl(aq) under the conditions of alloying with Mg, mixing with CuCl2 and heating at 773 K (500 °C). This result revealed that the some pretreatments enabled an efficient dissolution of Pt. Based on the methods investigated in this study, a new, environmentally friendly Pt recovery process can be developed.
References
S. Kondo, A. Takeyama, and T. Okura: Shigen-to-Sozai, 2006, vol. 122, pp. 386–95.
Y. Kita: Mineral Resources Report, JOGMEC, 2012, pp. 621–69.
R.T. Jones: JOM, 2004, vol. 56, pp. 59–63.
Anglo American Platinum: Group Performance Data. http://www.angloplatinum.com
J. Butler: Platinum 2011, Johnson Matthey Plc., London, U.K., 2011.
R.M. Heck and R.J. Farrauto: Appl. Catal. A, 2001, vol. 221, pp. 443–57.
T.H. Okabe: Kinzoku, 2006, vol. 76, pp. 980–84.
U.S. Geological Survey: Platinum-Group Metals Statistics and Information. http://minerals.usgs.gov/minerals/pubs/commodity/platinum/mcs-2011-plati.pdf.
Y. Tsugita: Kinzoku, 2006, vol. 76, pp. 991–97.
J. Nell: J. S. Afr. Inst. Min. Metal., 2004, vol. 104, pp. 423–28.
F. Habashi, ed.: Handbook of Extractive Metallurgy, vol. 3, Wiley-VCH, Weinheim, Germany, 1997.
F. Crundwell, M. Moats, V. Ramachandran, T. Robinson, and W.G. Davenport: Extractive Metallurgy of Nickel, Cobalt and Platinum-Group Metals, Elsevier, New York, NY, 2011.
N. Ezawa: Japanese Patent JPA1992-317423, 1992.
S.J. Al-bazi and A. Chow: Talanta, 1984, vol. 31, pp. 815–36.
E.R. Els, L. Lorenzen, and C. Aldrich: Minerals Eng., 2000, vol. 13, pp. 401–14.
O.N. Kononova, A.M. Melnikov, T.V. Borisova, and A.S. Krylov: Hydrometallurgy, 2011, vol. 105, pp. 341–49.
R.K. Mishra: Precious Metals 1993, IPMI, Flagstaff, AZ, 1993, pp. 449–74.
K. Gloe, P. Muhl, and M. Knothe: Hydrometallurgy, 1990, vol. 25, pp. 99–110.
T.H. Okabe, S. Yamamoto, Y. Kayanuma, and M. Maeda: J. Mater. Res., 2003, vol. 18, pp. 1960–67.
T.H. Okabe, Y. Kayanuma, S. Yamamoto, and M. Maeda: Mater. Trans. JIM, 2003, vol. 44, pp. 1386–93.
Y. Kayanuma, T.H. Okabe, Y. Mitsuda, and M. Maeda: J. Alloy. Compd., 2004, vol. 365, pp. 211–20.
Y. Kayanuma, T.H. Okabe, and M. Maeda: Metall. Mater. Trans. B, 2004, vol. 35B, pp. 817–24.
M. Pourbaix: Atlas of Electrochemical Equilibria in Aqueous Solution, Pergamon Press, Houston, TX, 1996.
J. Speight: Lange’s Handbook of Chemistry, 70th Anniversary ed., McGraw-Hill Professional, New York, NY, 2004.
N. Masuko: Denki Kagaku, 1959, vol. 27, pp. 365–74.
A. Yazawa and M. Eguchi: Shisshiki Seiren to Haisui Syori (Hydrometallurgy and Wastewater Treatment), Kyoritsu Shuppan Co., Tokyo, Japan, 1975, pp. 29–41.
The Chemical Society of Japan: Jikken Kagaku Koza (Encyclopedia of Experimental Chemistry), 5th ed., vol. 23 (Inorganic Compound), Maruzen Co., Tokyo, Japan, 2005.
Dictionary of Inorganic Compounds and Complexes, M. Nakahara, ed., Kodansha, Tokyo, Japan, 1997.
I. Barin: Thermochemical Data of Pure Substances, 3rd ed., VCH Publishers, Inc., Weinheim, Germany, 1995.
Acknowledgments
We are grateful to Professors Masafumi Maeda and Yoshitaka Mitsuda, Institute of Industrial Science of the University of Tokyo; Professors Yasuhiro Awakura (professor emeritus) and Tetsuya Uda, Graduate School of Engineering of Kyoto University; Dr. Kouji Yasuda (presently Graduate School of Energy Science of Kyoto University) and Dr. Katsuhiro Nose, Institute of Industrial Science of the University of Tokyo; and Ms. Chihiro Ohkawa, Graduate School of Engineering of the University of Tokyo (presently NEC Corp.) for fruitful discussions. We also thank Mr. Hisao Kimura, Institute of Industrial Science of the University of Tokyo, for his technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 12, 2012.
Rights and permissions
About this article
Cite this article
Horike, C., Morita, K. & Okabe, T.H. Effective Dissolution of Platinum by Using Chloride Salts in Recovery Process. Metall Mater Trans B 43, 1300–1307 (2012). https://doi.org/10.1007/s11663-012-9746-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-012-9746-z