Abstract
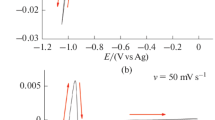
Voltammetry and chronoamperometry for the electrodeposition of tin from Tin(II) methane sulfonate mixed with ionic liquid and methane sulfonate acid at room temperature was studied. Cyclic voltammetry shows redox waves of Tin(II), which proves that the electrodeposition of tin from Tin(II) methane sulfonate is a diffusion-controlled process. The diffusion coefficient of Tin(II) ions in the solvent mixture showed good agreement from both voltammetry and chronoamperometry results. The diffusion coefficient of Tin(II) in the mixture was much smaller than in aqueous solution, and it depends on the anion of the ionic liquid.








Similar content being viewed by others
References
C.T.J. Low and F.C. Walsh: Electrochim. Acta, 2008, vol. 33, no. 16, pp. 5280-86.
J.F. Huang and I.W. Sun: J. Electrochem. Soc., 2003, vol. 150, no. 6, pp. E229-E306.
J.S. Wilkes and M.J. Zaworotko: J. Chem. Soc., Chem. Comm., 1992, (13), pp. 965–67.
S. Zein El Abedin, E.M. Moustafa, R. Hempelmann, H. Natter, and F. Endres: Electrochem. Comm., 2005, vol. 7, no. 11, pp. 1111-16.
A.P. Abbott, I. Dalrymple, F. Endres, and D.R. MacFarlane: Electrodeposition from Ionic Liquids, 1st ed. A.P. Abbott and D.R. MacFarlane, eds., Wiley-VCH, Weinheim, Germany, 2008, pp. 1–12.
C.L. Hussey and X.H. Xe: J. Electrochem. Soc., 1993, vol. 140, no. 3, pp. 618-26.
W.Z. Yang, H. Chang, Y.M. Tang, J.T. Wang, and Y.X. Shi: J. App. Electrochem., 2008, vol. 38, pp. 537-42.
N. Tachikawa, N. Serizawa, Y. Katayama, and T. Miura: Electrochim. Acta, 2008, vol. 53, pp. 6530-34.
A.J. Bard and L.R. Faulkner: Electrochemical Methods: Fundamentals and Applications, 2nd ed., Wiley, New York, NY, 2000, pp. 163, 231.
W.J. Basirun, D. Pletcher, A.Saraby Reintjes: J. App. Electrochem., 1996, vol. 26, pp. 873-80.
W.J. Basirun and D. Pletcher: J. App. Electrochem., 1998, vol. 28, pp. 167-72.
M. Ebadi, W.J. Basirun, and Y. Alias: Asian J. Chem., 2009, vol. 21, no. 8, pp. 6343-53.
M. Ebadi, W.J. Basirun, and Y. Alias: Asian J. Chem., 2009, vol. 21, no. 9, pp. 7354-62.
M. Ebadi, W.J. Basirun, Y. Alias, and M.R. Mahmoudian: Chem. Central. J., 2010, vol. 4, p. 14.
M. Ebadi, W.J. Basirun, and Y. Alias: J. Chem. Sci., 2010, vol. 122, no. 2, pp. 1-7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 4, 2011.
Rights and permissions
About this article
Cite this article
Yang, K.K., Mahmoudian, M.R., Ebadi, M. et al. Diffusion Coefficient of Tin(II) Methanesulfonate in Ionic Liquid and Methane Sulfonic Acid (MSA) Solvent. Metall Mater Trans B 42, 1274–1279 (2011). https://doi.org/10.1007/s11663-011-9560-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-011-9560-z