Abstract
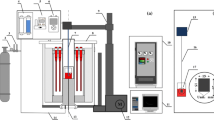
Density measurements of a low-silica CaO-SiO2-Al2O3 system were carried out using the Archimedes principle. A Pt 30 pct Rh bob and wire arrangement was used for this purpose. The results obtained were in good agreement with those obtained from the model developed in the current group as well as with other results reported earlier. The density for the CaO-SiO2 and the CaO-Al2O3 binary slag systems also was estimated from the ternary values. The extrapolation of density values for high-silica systems also showed good agreement with previous works. An estimation for the density value of CaO was made from the current experimental data. The density decrease at high temperatures was interpreted based on the silicate structure. As the mole percent of SiO2 was below the 33 pct required for the orthosilicate composition, discrete \( {\text{SiO}}_{4}^{4 - } \) tetrahedral units in the silicate melt would exist along with O2– ions. The change in melt expansivity may be attributed to the ionic expansions in the order of
Structural changes in the ternary slag also could be correlated to a drastic change in the value of enthalpy of mixing.






Similar content being viewed by others
Abbreviations
- T :
-
Temperature, K
- W argon :
-
Weight of the bob in argon atmosphere, g
- W molten slag :
-
Weight of the bob in molten slag, g
- V 0 :
-
Volume of the slag being displaced, cm3
- L T /L273:
-
Ratio in the change in length of a Pt 30 pct Rh wire at any temperature T (K) to the same at 273 K
- ρ expt :
-
Experimental density value, g/cm3
- R:
-
Gas constant, J/K mol
- X i :
-
Molar fractions of the individual oxides
- M i :
-
Molecular weights of the oxides, mol
- V mi :
-
Molar volumes of the oxides given as the ratio of the molecular weight by the density of the oxide, cm3/mol
- H m :
-
Relative integral molar enthalpy of mixing derived by solving the Gibbs–Helmoltz equation for the relative integral molar Gibbs energy of mixing. The enthalpy thus derived is a function of the ionic fractions of cations and anions in their respective grouping and the interaction variables derived for various binary oxide systems and temperature, J/mol
- λ :
-
Constant and is evaluated for binary systems using experimental density data in literature
References
M. Persson, J. Zhang, and S. Seetharaman: Steel Res. Int., 2007, vol. 78, no. 4, pp. 290-98.
Royal Institute of Technology: Thermoslag, Division of Materials Process Science, Royal Institute of Technology, Stockholm, Sweden, 1996.
M. Persson, T. Matsushita, J. Zhang, and S. Seetharaman: Steel Res. Int., 2007, vol. 78, no. 2, pp. 102-08.
Y.E. Lee and D.R. Gaskell: Metall. Trans., 1974, vol. 5, pp. 853-74.
Y. Sato, T. Nishizuka, K. Hara, T. Yamamura, and Y. Waseeda: Int. J. Thermophys., 2000, vol. 21, no. 6, pp. 1463-71.
T. Matsushita, T. Ishikawa, P.F. Paradis, K. Mukai, and S. Seetharaman: ISIJ Int., 2006, vol. 46, no. 4, pp. 606-10.
A.I Sergienko: Avt. Svarka, 1965, vol. 18, no. 6, pp. 26-31.
T. Takayanagi, M. Kato, and S. Minowa: J. Jap. Foundry Soc., 1976, vol. 48, no. 12, pp. 779-83.
S. Hara, K. Irie, D.R. Gaskel, and K. Ogino: Trans. JIM, 1988, vol. 29, pp. 977-89.
E.V. Krinochkin, K.T. Kurochin, P.V. Umrikhin, F.K. Poverkh, and Y. Rasp: Ed. V.N. Eremenko, Naukova Dumka, Kiev, Ukraine, 1971, pp. 179–83.
H. Winterhager, L. Greiner, and R. Kamnel: Forschungsberichte des Landes Nordrhein-Westfalen, no. 1630, Westdeutscher Verlag, Germany, 1966.
L. R. Barett and A.G. Thomas: J. Glass Technol., 1959, vol. 43, p. 179.
A.I. Bochorishvili and S.B Yakobashvili: Svar. Proiz., 1968, vol. 10, pp. 13-15.
M. Zieliński and B. Sikora: Pr. Inst. Metall. Zelaza im St. Staszica, 1977, vol. 29, nos. 3-4, pp. 229-32.
Verein Deutscher Eisenhűttenleute: Slag Atlas, Verlag Stahleisen m.b.h, Dusseldorf, Germany, 1981, p. 57.
A. Jakobsson, N.N. Vishwanathan, S. Du, and S. Seetharaman: Metall. Mater. Trans. B, 2000, vol. 31B, p. 974.
B. Barter and A.S. Darling: Platinum Metals Rev., 1960, vol. 4, pp. 138-40.
Verein Deutscher Eisenhűttenleute: Slag Atlas, Verlag Stahleisen m.b.h, Dusseldorf, Germany, 1981, pp. 239.
G. Parry and O. Ostrovski: Metall. Mater. Trans. B, 2008, vol. 39B, pp. 681-89.
S.B. Yakobashvili: Auto Weld, 1970, vol. 23, no. 6, pp. 20.
V.I. Sokolov, S.I. Popel, and O.A. Esin: Černaja Metallugija, 1970, vol. 13, no. 2, pp. 10-15.
B.S. Mitin and Yu. A. Nagibin: Russ. J. Phys. Chem., 1970, vol. 44, no. 5, pp. 741-42.
J.F. Bacon, A.A. Hasapis, and J.W. Wholley: J. Phys. Chem. Glasses, 1960, vol. 1, pp. 90-98.
F.D. Richardson: Trans. Faraday Soc. 1956, vol. 52, p. 1312.
C.J.B. Fincham and F.D. Richardson: Proc. Roy. Soc., 1954, vol. 223A, p. 40.
Y. Waseda and J.M. Touguri: The Structure and Properties of Oxide Melts, World Scientific, Hackensack, NJ, 1998, pp. 4.
S. Seetharaman and L.I. Staffansson: Chemica Scripta, 1976, vol. 10, pp. 61-66.
P. Kubaschewski and C.B. Alcock: Thermochimie, Colloq. Internat. Centre National Recher. Scientif., No. 201, Marseille, Paris, 1972, France, p. 211.
Acknowledgments
The authors would like to thank the Swedish Research Council (Project No: H 6971) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 17, 2009.
Rights and permissions
About this article
Cite this article
Muhmood, L., Seetharaman, S. Density Measurements of Low Silica CaO-SiO2-Al2O3 Slags. Metall Mater Trans B 41, 833–840 (2010). https://doi.org/10.1007/s11663-010-9385-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-010-9385-1