Abstract
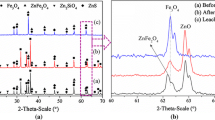
The addition of secondary additives, such as CaCO3 and MnCO3, during the Na2CO3 roasting of La Oroya zinc ferrite residue (19.5 pct Zn, 26.6 pct Fe) to try to control iron dissolution during subsequent leaching with 200 g/L H2SO4 was examined. Design of experiments (DOE) testing was used to study the effects of temperature, Na2CO3, and CaCO3/MnCO3 on metals extractions, the phases formed during roasting, and the phases remaining after leaching. Increased temperature and Na2CO3 additions during roasting increased zinc extractions during leaching with maximum zinc extractions at 950 °C, 80 pct Na2CO3, and the lowest CaCO3 or MnCO3 addition tested. However, the addition of 29.5 pct CaCO3 or 33.3 pct MnCO3 reduced iron extractions by 20 and 40 pct, respectively, while maintaining zinc extractions of over 90 pct. Mineralogical analysis using X-ray diffraction (XRD) and scanning electron microscopy/energy dispersive X-ray (SEM/EDX) analysis showed ZnO, α-NaFeO2, β-NaFeO2, Na2ZnSiO4, and Na2CO3/Na2O/Na2SO4 as major phases, but also showed evidence for the formation of Ca- or Mn-ferrites during roasting. The srebrodolskite (Ca2Fe2O5) phase formed during roasting with CaCO3 was partially soluble during acid leaching, while the Mn-Fe oxides formed during roasting with MnCO3 were insoluble during acid leaching. The formation of these metal ferrites is believed to be the source of the reduced iron extractions observed for roasting with these additives.















Similar content being viewed by others
Notes
FREED is a trademark of Thermart, San Diego, CA, USA.
FACTSAGE is a trademark of Thermafact/CRCT, Montreal, Quebec, Canada and GTT-Technologies, Aachen, Germany.
DOE XL PRO is a trademark of Digital Computations, Inc. and Air Academy Associates, LLC, Colorado Springs, Colorado, USA.
PERKIN-ELMER is a trademark of Perkin-Elmer, Wellesley, MA.
HITACHI is a trademark of Hitachi High-Technologies Canada, Inc., Rexdale, ON, Canada.
GW ELECTRONICS is a trademark of EBSciences, East Granby, CT.
PRINCETON GAMMA TECH is a trademark of Princeton Gamma Tech, Princeton, NJ.
RIGAKU is a trademark of Rigaku Americas Corporation, The Woodlands, TX.
JADE is a trademark of Materials Data, Incorporated, Livermore, CA.
References
D.W. Hopkins: Bull. Inst. Min. Metall., 1949, vol. 515, pp. 1–21
P.C. Holloway and T.H. Etsell: Mineral Processing and Extractive Metallurgy (Trans. IMM C), 2007, vol. 116, in press-a
P.C. Holloway, T.H. Etsell, and A.L. Murland: Metall. Mater. Trans. B, 2007, vol. 38B, in press
C.E. Swartz, F.C. Krauskopf: Techn. Publ. Am. Inst. Min. Metall. Eng., 1927, vol. 40, pp. 1–19
R. Dimitrov, B. Boyanov: Rud. Metal. Zb., 1984, vol. 31, pp. 67–80
M.B. Morsi, M.M. Nasr, F.H.A. Abdalla, S.Z. El-Tawil: Trans. Indian Inst. Met., 1998, vol. 51 (4), pp. 193–200
D.K. Xia, C.A. Pickles: Can. Metall. Q., 2000. vol. 38 (3), pp. 175–86
Z. Youcai, R. Stanforth: J. Haz. Mater., 2000, vol. B80, pp. 223–40
J. Reyes: Doe Run Peru, La Oroya, Peru, private communication, 2004
J. Chadwick: J. Min. Mag., 1998, vol. 178 (2), pp. 106 and 108–11
U.S. Geological Survey: Mineral Commodity Summaries 2007, U.S. Geological Survey, Washington, DC, 2007, pp. 102–03
J. Sofra, A. Heinz: Eur. Metallurgical Conf. 2003 vol. 2, GDMB Medienverlag, Clausthal-Zellerfeld, Germany, 2003, pp. 491–506
P.C. Holloway and T.H. Etsell: Mineral Processing and Extractive Metallurgy (Trans. IMM C), 2007, vol. 116, in press-b
Acknowledgments
The authors thank Doe Run Peru for supplying a sample of zinc ferrite residue for metallurgical testing. The authors also thank the Natural Sciences and Engineering Research Council (NSERC) of Canada (NSERC) and Alberta Ingenuity for supplying student funding to help support this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted February 11, 2007.
Rights and permissions
About this article
Cite this article
Holloway, P., Etsell, T. & Murland, A. Use of Secondary Additives to Control the Dissolution of Iron during Na2CO3 Roasting of La Oroya Zinc Ferrite. Metall Mater Trans B 38, 793–808 (2007). https://doi.org/10.1007/s11663-007-9083-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-007-9083-9