Abstract
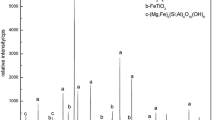
In this article, a study of the thermodynamics of reduction of titanium-magnetite concentrate with solid carbon in different stoichiometric proportions (carbon-concentrate 1:1, 2:1 and 2.5:1) in the temperature range of 973 to 1273 K is presented. The Gibbs free energy ΔG° T = f(T), log10 P CO 2/P CO = f(T), and electromotive force (EMF) = f(T) of the probable reaction Fe3O4 + C = 3FeO + CO in the concentrate is studied by the galvanic cell with different reference electrode-air and Ni/NiO. The X-ray analysis indicates different behaviors of the reduction process with solid carbon when 1:1, 2:1, and 2.5:1 ratios of carbon/concentrate were used. The best result is obtained when the ratio is 2.5:1. Some amorphous mass (diatomite) is also observed. The highest concentration of CO (62 to 65 pct) in the gas phase was observed at a carbon-to-concentrate ratio 2:1 and the lowest concentration of CO (40 to 45 pct) at the ratio 1:1.
Similar content being viewed by others
References
P.J. Spencer and O. Kubaschewski: CALPHAD, Pergamon Press Limited, United Kingdom, 1978, vol. 2, pp. 147–67.
H.F. Rizzo, R.S. Gordon, and I.B. Cutler: J. Electrochem. Soc: Solid State Sci., 1969, vol. 116 (2), pp. 266–74.
R.A. Giddings and R.S. Gordon: J. of Am. Ceram. Soc., 1973, vol. 56 (3), pp. 111–16.
K. Kiukkola and C. Wagner: J. Electrochem. Soc., 1957, vol. 104 (6), pp. 379–86.
M. Farren, S.P. Matthew, and P.C. Hayes: Metall. Trans. B, 1990, vol. 21B, pp. 135–39.
K. Kashida, K. Goto, and M. Someno: Trans. TMS-AIME, 1968, vol. 242 (1), pp. 82–87.
K. Ono and J. Moriyama: J. Mining Metall. Inst. Jpn., 1973, vol. 89 (1023), pp. 313–16.
S. Kang and Z. Lei: Trans. Nonferrous Met. Soc. China, 1996, vol. 6 (1), pp. 25–31.
Z. Moser, K. Fitzner, and W. Zakulski: Bulletin Acad. Polonaise Sci., 1975, vol. 23 (3), pp. 243–48.
E. Jacobsson and E. Rosen: Scand. J. Metall., 1981, vol. 10, pp. 39–43.
O. Sjoden, S. Seetharaman, and L.I. Staffansson: Metall. Trans., 1986, vol. 17B, pp. 179–84.
P. Vallet and P. Raccah: Mem. Sci. Rev. Met., 1965, vol. 62, p. 1.
M.N. Karapetjantz: Chimicheskaja Termodynamika, Chimia, Moscow, 1975, pp. 537–53.
S.I. Philippov: Theorie of Metallurgical Processes, Metallurgy, Moscow, 1967, p. 17.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paunova, R. Thermodynamic study of the reduction of titanium magnetite concentrate with solid carbon. Metall Mater Trans B 33, 633–638 (2002). https://doi.org/10.1007/s11663-002-0043-0
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11663-002-0043-0