Abstract
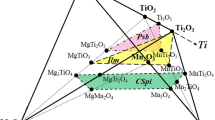
An experimental study of the PbO-MgO-SiO2 system has been carried out using high-temperature equilibration and quenching techniques, followed by electron probe X-ray microanalysis (EPMA). The phase equilibria were determined in the temperature range from 973 to 1673K. Nine primary phase fields have been investigated in this system, including three monoxides (PbO, MgO, and SiO2), five binary compounds (MgSiO3, Mg2SiO4, PbSiO3, Pb2SiO4, and Pb4SiO6), and one ternary compound Pb8Mg(Si2O7)3. Two other ternary compounds, PbMgSiO4 and PbMgSi3O8, were observed in some experiments; however, further experiments indicated that these two compounds are unstable in the temperature range investigated.
Similar content being viewed by others
References
J.W. Greig: J. Am. Sci., 1927, 5th ser. vol. 13, pp. 1–154.
N.L. Bowen and O. Andersen: Am. J. Sci., 1914, vol. 37, pp. 487–500.
C.M. Schlaudt and D.M. Roy: J. Am. Ceram. Soc., 1965, vol. 48, pp. 248–51.
P. Wu, G. Eriksson, A.D. Pelton, and M. Blander: Iron Steel Inst. Jpn. J., 1993, vol. 33, pp. 25–34.
R.M. Smart and F.P. Glasser: J. Am. Ceram. Soc., 1974, vol. 57, pp. 378–82.
R.F. Geller, A.S. Creamer, and E.N. Bunting: Research Paper No. RP705, National Bureau of Standards, Gaithersburg, MD, 1934, vol. 13, pp. 237–44.
U. Kuxmann and P. Fischer: Erzmetallurgy, 1974, vol. 27, pp. 533–37.
P.D. Clavert and R.R. Shaw: J. Am. Ceram. Soc., 1970, vol. 53, pp. 350–52.
K.A. Krakau, E.J. Mukhin, and M.S. Geinrich: Fiziko-Khimicheskie Svoistva Troinoi sisteyemy: Okis Natriya-Okis Svintsa-kremnezem, Grebenshchikov, Moscow, 1949, pp. 15–38.
E. Jak, S. Degterov, P. Wu, P.C. Hayes, and A.D. Pelton: Metall. Mater. Trans. B, 1997, vol. 28B, pp. 1011–18.
J.F. Argyle and F.A. Hummel: Glass Industry, 1965, Dec., pp. 710–18.
H.W. Billhardt: Am. Mineral., 1969, vol. 54, pp. 510–21.
E. Sugimoto and Z. Kosuka: Trans. Jpn. Inst. Met., 1978, vol. 19, pp. 275–80.
E. Jak, N. Liu, and P.C. Hayes: Metall. Mater. Trans. B, 1998, vol. 29B, pp. 541–53.
E. Jak, S. Degterov, P.C. Hayes, and A.D. Pelton: Can. Metall. Q., 1998, vol. 37, pp. 41–47.
E. Jak, H.G. Lee, and P.C. Hayes: Kor. IMMJ, 1995, vol. 1, pp. 1–8.
E. Jak, S. Degterov, B. Zhao, A.D. Pelton, and P.C. Hayes: Proc. Zinc and Lead Processing Symp., Calgary, CIM, Montreal, 1998, pp. 313–33.
D.R. Lide: CRC Handbook of Chemistry and Physics, 81st ed., CRC Press, Boca Raton, FL, 2000, pp. 4–50 and 70.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, S., Zhao, B., Jak, E. et al. Experimental study of phase equilibria in the PbO-MgO-SiO2 system. Metall Mater Trans B 32, 11–16 (2001). https://doi.org/10.1007/s11663-001-0002-1
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11663-001-0002-1