Abstract
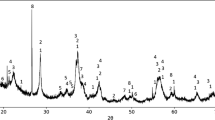
A step has been made in the direction of understanding the fundamental chemical processes taking place inside electric are furnaces producing manganese alloys. The reduction of higher manganese oxides to MnO by carbon monoxide has been studied in the temperature range 700 °C to 1100 °C. A topochemical pattern with a single shrinking core inside the ore particles has been observed in most cases. It has been found that the reduction of some manganese silicates (braunite minerals) is influenced by reaction interface kinetics, whereas the reduction rate of manganese oxides (bixbyite and hausmannite) is mostly determined by product shell pore diffusion. Sintering kinetics and the extent of natural porosity determine the product shell pore diffusivity. As the melting point of the reaction product is approached, rapid sintering leads to a decrease in diffusivity.
Similar content being viewed by others
References
M. Tangstad and S.E. Olsen: Proc. INFACON 7, The Research Association of Norwegian Ferroalloy Producers, Trondheim, Norway, 1995, pp. 621–30.
D.A. Aderibigbe and J. Szekely: Ironmaking and Steelmaking, 1981, vol. 8, pp. 11–19.
A.W.D. Hills: Chem. Eng. Sci., 1968, vol. 23, pp. 297–320.
H.E. Barner and C.L. Mantell: Ind. Eng. Chem. Process Des. Development, 1968, vol. 7, pp. 285–94.
T.J.W. de Bruijn, T.H. Soerawidjaja, W.A. de Jong, and P.J. van den Berg: Chem. Eng. Sci., 1980, vol. 35, pp. 1591–1600.
V.N. Misra, R.C. Sinvhal, and P.R. Khangaonkar: Trans. Ind. Inst. Met., 1975, vol. 28, pp. 268–69.
J. Szekely, J.W. Evans, and H.Y. Sohn: Gas-Solid Reactions, Academic Press, New York, NY, 1976, pp. 256–86.
A.S.E. Kleyenstuber: Trans. Geol. Soc. South Africa, 1984, vol. 87, pp. 257–72.
W.F. Frazer and C.B. Belcher: Proc. Australasian Institute of Mining and Metallurgy, Australasian Inst. of Mining and Metallurgy, Victoria, Australia, 1975, pp. 29–36.
K.L. Berg: Ph.D. Thesis, NTNU, Trondheim, Norway, 1998, p. 37, 110.
C.J. Geankoplis: Transport Processes and Unit Operations, 2nd ed., Allyn and Bacon, Newton, MA, 1983, pp. 433–36.
M. Wang and B. Sundman: Metall. Trans. B, 1992, vol. 23B, pp. 821–31.
R.H. Spitzer, F.S. Manning, and W.O. Philbrook: TMS-AIME, 1966, vol. 236, pp. 1715–24.
W.A. Oates and D.D. Todd: J. Austr. Inst. Met., 1962, vol. 7, pp. 109–14.
R.B. Bird, W.E. Stewart, and E.N. Lightfoot: Transport Phenomena, John Wiley & Sons, New York, NY, 1960, p. 511.
C.J. Geankoplis: Transport Processes and Unit Operations, 2nd ed., Allyn and Bacon, Newton, MA, 1983, p. 452.
H.O. Lien, A.E. El-Mehairy, and H.U. Ross: J. Iron Steel Inst., 1971, vol. 209, pp. 541–45.
F.P. Glasser: J. Am. Ceram. Soc., 1962, vol. 45, p. 245.
F.P. Glasser: Am. J. Sci., 1958, vol. 256, p. 405.
W.M. McKewan: TMS-AIME, 1960, vol. 218, pp. 2–6.
J. Szekely and J.W. Evans: Metall. Trans., 1971, vol. 2, pp. 1699–1710.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berg, K.L., Olsen, S.E. Kinetics of manganese ore reduction by carbon monoxide. Metall Mater Trans B 31, 477–490 (2000). https://doi.org/10.1007/s11663-000-0154-4
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11663-000-0154-4