Abstract
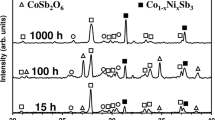
The skutterudite materials belonging to the CoSb3 family are widely studied for thermoelectric applications. They are typically used under vacuum, but applications under oxidizing environments are increasingly considered. The addition of ytterbium is known to enhance the thermoelectric properties of CoSb3, but the corresponding impact on the oxidation behavior of the skutterudite material is barely explored. For that purpose, the present research describes the oxidation behavior of Yb0.2Co4Sb12 under a flow of air at 800 K for 15, 50, 100, and 1000 hours. The oxidation treatment induces the growth of a surface layer made of three oxides in various amounts as a function of the oxidation time. The spinel oxide CoSb2O4/CoO·Sb2O3 and CoSb2O6 are observed from 15 hours of oxidation, whereas Sb2O4 is formed only from 100 hours of treatment. To assess the impact of ytterbium, the oxidation behavior of Yb0.2Co4Sb12 is compared to that of CoSb3 oxidized in the same experimental conditions. The results show that the low amount of ytterbium promotes the oxidation reactions of the skutterudite material. Nonetheless, the impact on the degradation of the material remains acceptable to use Yb0.2Co4Sb12 for thermoelectric applications under oxidizing environments.











Similar content being viewed by others
References
M. Rull-Bravo, A. Moure, J.F. Fernandez, and M. Martin-Gonzalez: RSC Adv., 2015, vol. 5, pp. 41653-67.
G. Rogl and P. Rogl: Curr. Opin. Green Sustain. Chem., 2017, vol. 4, pp. 50-7.
R. Drevet, L. Aranda, C. Petitjean, N. David, D. Veys–Renaux, and P. Berthod: Oxid. Met., 2019, vol. 91, pp.767-79.
W. Liu, Q. Jie, H.S. Kim, and Z. Ren: Acta Mater., 2015, vol. 87, pp. 357-76.
M. Zebarjadi, K. Esfarjani, M.S. Dresselhaus, Z.F. Ren, and G. Chen: Energy Environ. Sci., 2012, vol. 5, pp. 5147-62.
J.W. Sharp, E.C. Jones, R.K. Williams, P.M. Martin, and B.C. Sales: J. Appl. Phys., 1995, vol. 78, pp. 1013-18.
J.X. Zhang, Q.M. Lu, K.G. Liu, L. Zhang, and M.L. Zhou: Mater. Lett., 2004, vol. 58, pp. 1981-4.
Y. Kawaharada, K. Kurosaki, M. Uno, and S. Yamanaka: J. Alloys Compd., 2001, vol. 315, pp. 193-7.
R. Guo, X. Wang, and B. Huang: Sci. Rep., 2015, vol. 5, 7806.
X. Shi, J. Yang, J.R. Salvador, M. Chi, J.Y. Cho, H. Wang, S. Bai, J. Yang, W. Zhang, and L. Chen: Multiple-Filled Skutterudites: J. Am. Chem. Soc., 2011, vol. 133, pp. 7837-46.
E. Alleno, E. Zehani, and O. Rouleau: J. Alloys Compd., 2013, vol. 572, pp. 43-8.
E. Alleno, E. Zehani, M. Gaborit, V. Orodniichuk, B. Lenoir, and M. Benyahia: J. Alloys Compd., 2017, vol. 692, pp. 676-86.
T. Dahal, Q. Jie, G. Joshi, S. Chen, C. Guo, Y. Lan, and Z. Ren: Acta Mater., 2014, vol. 75, pp. 316-21.
X. Xia, P. Qiu, X. Shi, X. Li, X. Huang, and L. Chen: J. Electron. Mater., 2012, vol. 41, pp. 2225-31.
I.K. Dimitrov, M.E. Manley, and S. Shapiro: Phys. Rev. B, 2010, vol. 82, 174301.
J. Yang, Q. Hao, H. Wang, Y. C. Lan, Q.Y. He, A.J. Minnich, D.Z. Wang, J.A. Harriman, V.M. Varki, M. Dresselhaus, G. Chen, and Z.F. Ren: Phys. Rev. B, 2009, vol. 80, 115329.
L.E. Bell: Science, 2008, vol. 321, pp. 1457-61.
V. Andrei, K. Bethke, and K. Rademann: Energy Environ. Sci., 2016, vol. 9, pp. 1528-32.
R. Drevet, L. Aranda, C. Petitjean, D. Veys-Renaux, N. David, and P. Berthod: Oxid. Met., 2020, vol. 93, pp. 559-72.
R. Kühn, O. Koeppen, P. Schulze, and D. Jänsch: Mater. Today proc., 2015, vol. 2, pp. 761-9.
D. Veys-Renaux, R. Drevet, C. Petitjean, L. Aranda, N. David, and P. Berthod: J. Solid State Electrochem., 2018, vol. 22, pp. 2821-8.
R. Drevet, C. Petitjean, N. David, L. Aranda, D. Veys-Renaux, and P. Berthod: Mater. Chem. Phys., 2020, vol. 241, 122417.
R. Drevet, L. Aranda, N. David, C. Petitjean, D. Veys-Renaux, and P. Berthod: Surf. Coat. Technol., 2020, vol. 385, 125401.
A. Navrotsky and O.J. Kleppa: J. Inorg. Nucl. Chem., 1968, vol. 30, pp. 479-98.
H.S.C. O’Neill and A. Navrotsky: Am. Mineral., 1983, vol. 68, pp. 181-94.
E. Godlewska, K. Zawadzka, A. Adamczyk, M. Mitoraj, and K. Mars: Oxid. Met., 2010, vol. 74, pp. 113-24.
J. Leszczynski, K.T. Wojciechowski, and A.L. Malecki: J. Therm. Anal. Calorim., 2011, vol. 105, pp. 211-22.
M.K. Kumawat, C. Parlikar, M.D. Zafir Alam, and D.K. Das: Metall. Mater. Trans. A, 2021, vol. 52A, pp. 378-93.
E. Mohammadi Zahrani and A.M. Alfantazi: Metall. Mater. Trans. A, 2013, vol. 44A, pp. 4671-99.
W.J. Zhang and R. Sharghi-Moshtaghin: Metall. Mater. Trans. A, 2021 vol. 52A, pp. 1492-1502.
R. Hara, S. Inoue, H.T. Kaibe, and S. Sano: J. Alloys Compd., 2003, vol. 349, pp. 297-301.
M. Ritouet and P. Berthod: Oxid. Met., 2018, vol. 89, pp. 339-55.
F. Wu, Q. He, D. Hu, F. Gao, H. Song, J. Jia, and X. Hu: J. Electron. Mater., 2013, vol. 42, pp. 2574-81.
W.L. Bragg: Philos. Mag., 1920, vol. 40, pp. 169-89.
P. Berthod and Z. Himeur: Oxid. Met., 2018, vol. 90, pp. 187-202.
V. Savchuk, A. Boulouz, S. Chakraborty, J. Schumann, and H. Vinzelberg: J. Appl. Phys., 2002, vol. 92, pp. 5319-26.
Acknowledgments
The French National Research Agency (ANR) is gratefully acknowledged for the financial support of the Nanoskut Project (ANR-12-PRGE-0008-01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted on December 31, 2020; accepted June 8, 2021.
Rights and permissions
About this article
Cite this article
Drevet, R., Aranda, L., David, N. et al. Oxidation Behavior of the Skutterudite Material Yb0.2Co4Sb12. Metall Mater Trans A 52, 3996–4002 (2021). https://doi.org/10.1007/s11661-021-06359-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-021-06359-6