Abstract
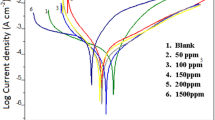
Several compounds tested as corrosion inhibitors have proven to possess good effectiveness in protection of steel rebar in concrete. However, most of them are considered as pollutant compounds, which limits their use. The aim of this work is to investigate the inhibitive effect of sodium molybdate, which is considered as a nonpollutant compound, against pitting corrosion of steel rebar in simulated concrete pore solution. Corrosion behaviors of steel in different solutions were studied by means of corrosion potential, potentiodynamic polarization, and electrochemical impedance spectroscopy. The results indicate that the addition of sodium molybdate to the chlorinated solution decreases significantly the corrosion rate of steel. Due to its passivating character, the sodium molybdate inhibitor promotes the formation of a stable passive layer on the surface of steel, acting as a physical barrier against chloride ions, on one hand, and consolidating the passivation mechanism of steel, on the other. The optimal inhibition rate is given by the concentration of molybdate ions, corresponding to a [MoO4 2−]/[Cl−] that is equal to 0.5.














Similar content being viewed by others
References
H. Bensabra and N. Azzouz: Metall. Mater. Trans. A, 2013, vol. 44A, pp. 5703–10.
L. Yohai, M. Vázquez, and M.B. Valcarce: Electrochim. Acta, 2013, vol. 102, pp. 88–96.
M.F. Montemor, A.M.P. Simões, and M.G.S. Ferreira: Cem. Conc. Comp., 2003, vol. 25, p. 491.
X. Shi, N. Xie, K. Fortune, and J. Gong: Const. Build. Mater., 2012, vol. 30, p. 125.
M. Moreno, W. Morris, M.G. Alvarez, and G.S. Duffo: Corr. Sci., 2004, vol. 46, p. 2681.
M.B. Valcarce and M. Vázquez: Mater. Chem. Phys., 2006, vol. 115, p. 313.
F. Fei, J. Hu, J. Wei, Q.J. Yu, and Z. Chen: Const. Building Mater., 2014, vol. 70, pp. 43–53.
J. Hu, D. Koleva, J. De Wit, H. Kolev, and K. Van Breugel: J. Electrochem. Soc., 2011, vol. 158, pp. 76–87.
J.J. Assaada and C. Issa: Const. Building Mater., 2012, vol. 30, pp. 667–74.
S.R. Yeomans: Corrosion, 1994, vol. 50, pp. 72–81.
A.M.G. Seneviratne, G. Sergi, and C.L. Page: Constr. Building Mater., 2000, vol. 14, pp. 55–59.
H. Bensabra, N. Azzouz, and J.P. Chopart: Rev. Métall., 2012, vol. 109, pp. 167–75.
T.A. Söylev and M.G. Richardson: Constr. Building Mater., 2008, vol. 22 (4), pp. 609–22.
M. Ormellese, F. Bolzoni, S. Goidanich, M.P. Pedeferri, and A. Brenna: Corros. Eng. Sci. Technol., 2011, vol. 46 (4), pp. 334–39.
W. Morris, A. Vico, and M. Vázquez: J. Appl. Electrochem., 2003, vol. 33, pp. 1183–89.
D.M. Bastidas, M. Criado, V.M. La Iglesia, S. Fajardo, A. La Iglesia, and J.M. Bastidas: Cem. Conc. Comp., 2013, vol. 43, pp. 31–38.
Ha-Won Song and Velu Saraswathy: Met. Mater. Int., 2006, vol. 12 (4) pp. 323–29.
L. Dhouibi, E. Triki, M. Salta, P. Rodrigues, and A. Raharinaivo: Mater. Struct., 2003, vol. 36, pp. 530–40.
J. Shi and W. Sun: Int. J. Miner. Metall. Mater., 2012, vol. 19(1), p. 38.
S. NealBerke and M.C. Hicks: Cem. Concr. Compos., 2004, vol. 26, pp. 191–98.
M. Cabrini, S. Lorenzi, and T. Pastore: Electrochim. Acta, 2014, vol. 124, pp. 156–64.
L. Dhouibi, P. Refait, E. Triki, and J.M.R. Génin: J. Mater. Sci., 2006, vol. 41, pp. 4928–36.
J.T. Hinatsu, W.F. Graydon, and F.R. Foulkes: J. Appl. Electrochem., 1990, vol. 20, pp. 840–47.
O.A. Omotosho, J.O. Okeniyi, and O.O. Ajayi: J. Fail. Anal. Preven., 2010, vol. 10, pp. 408–15.
Yuming Tang, Guodong Zhang, and Yu Zuo: Constr. Building Mater., 2012, vol. 28, pp. 327–32.
C.M. Hansson, L. Mammoliti, and B.B. Hope: Cem. Concr. Res., 1998, vol. 28, pp. 1775–78.
J.J. Shi, M. Saremi, and E. Mahallati: Cem. Concr. Res., 2002, vol. 32, pp. 1915–20.
C. Andrade, M. Keddam, X.R. Nóvoa, M.C. Pérez, C.M. Rangel, and H. Takenouti: Electrochim. Acta, 2001, vol. 46, pp. 3905–12.
S. Joiret, M. Keddam, X.R. Nóvoa, M.C. Pérez, C. Rangel, and H. Takenouti: Cem. Conc. Comp., 2001, vol. 24, pp. 7–15.
Jin-jie Shi and Wei Sun: Int. J. Miner. Metall. Mater., 2012, vol. 19 (1), pp. 8–17.
M.B. Valcarce and M. Vázquez: Electrochim. Acta, 2008, vol. 53 (15), p. 5007.
J.Y. Zhong, M. Sun, D.B. Liu, X.G. Li, and T.Q. Liu: Int. J. Miner. Metall. Mater., 2010, vol. 17, p. 282.
WH Zhang, SW Yang, J Guo, ZY Liu, and XL He: Int. J. Miner. Metall. Mater., 2010, vol. 17(6), p. 748.
M. Nagayama and M. Cohen: J. Electrochem. Soc., 1963, vol. 110, pp. 670–80.
J. Kruger and J.P. Calvert: J. Electrochem. Soc., 1967, vol. 114, pp. 43–49.
T. Yonezawa and V. Ashworth: Corrosion, 1988, Vol. 44, pp. 489–99.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 26, 2015.
Rights and permissions
About this article
Cite this article
Bensabra, H., Franczak, A., Aaboubi, O. et al. Inhibitive Effect of Molybdate Ions on the Electrochemical Behavior of Steel Rebar in Simulated Concrete Pore Solution. Metall Mater Trans A 48, 412–424 (2017). https://doi.org/10.1007/s11661-016-3778-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-016-3778-y