Abstract
In this study, passivation behavior of ultrafine-grained (UFG) pure copper fabricated by ARB process in 0.01 M borax solution has been investigated. Before any electrochemical measurements, evaluation of microstructure was obtained by transmission electron microscopy (TEM). TEM observations revealed that with increasing the number of ARB passes, the grain size of specimens decrease. Also, TEM images showed that UFGs with average size of below 100 nm appeared after 7 passes of ARB. To investigate the passivation behavior of the specimens, electrochemical impedance spectroscopy (EIS) and Mott-Schottky analysis was carried out. For this purpose, three potentials within the passive region were chosen for potentiostatic passive film growth. EIS results showed that both passive film and charge-transfer resistance increases with increasing the number of ARB passes. Moreover, Mott-Schottky analysis revealed that with increasing the number of ARB passes, the acceptor density of the passive films decreased. In conclusion, increasing the number of ARB passes offers better conditions for forming the passive films with higher protection behavior, due to the growth of a much thicker and less defective films.










Similar content being viewed by others
References
S.H. Sanad, and A.R. Taman: Surf. Tech., 1984, vol. 23, pp. 159–66.
Q. Zhong, L. Yu, Y. Xiao, Y. Wang, Q. Zhou, and Q. Zhong: Advanc. Mater. Res., 2013, vol. 785, pp. 928–32.
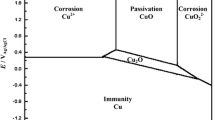
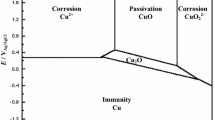
M. Pourbaix: Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed., NACE, Houston, 1974, pp. 85–87.
R.M. Souto, S. Gonzalez, R.C. Salvarezza, and A.J. Arvia: Electrochim. Acta., 1994, vol. 39, pp. 2619–28.
M. Raei, M.R. Toroghinejad, R. Jamaati, and J.A. Szpunar: Mater. Sci. Eng. A., 2010, vol. 527, pp. 7068–73.
R. Jamaati, M.R. Toroghinejad, and H. Edris: Mater. Sci. Eng. A., 2013, vol. 578, pp. 191–96.
S. Pasebani, and M.R. Toroghinejad: Mater. Sci. Eng. A., 2010, vol. 527, pp. 491–97.
S. Pasebani, and M.R. Toroghinejad, M. Hosseini, J. Szpunar:, Mater. Sci. Eng. A., 2010, vol. 527, pp. 2050–56.
W. Wei, K. X. Wei, and Q. B. Du: Mater. Sci. Eng. A., 2007, vol. 454, pp. 536–41.
M. Kadkhodaee, M. Babaiee, H. DaneshManesh, M. Pakshir, and B. Hashemi: J. Alloys Comp., 2013, vol. 576, pp. 66–71.
M.R. Rezaei, M.R. Toroghinejad, and F. Ashrafizadeh: J. Mater. Process. Tech., 2011, vol. 211, pp. 1184–90.
E. Darmiani, I. Danaee, M.A. Golozar, and M.R. Toroghinejad: J. Alloys Comp., 2013, vol. 552, pp. 31–39.
Y. Saito, H. Utsunomiya, N. Tsuji, and T. Sakai: Acta Mater., 1999, vol. 47, pp. 579–83.
R. Jamaati, M.R. Toroghinejad, and A. Najafizadeh: Mater. Sci. Eng. A., 2010, vol. 527, pp. 3857–63.
S.A. Hosseini, and H. DaneshManesh: Mater. Des., 2009, vol. 30, pp. 2911–18.
A. Fattah-alhosseini, and O. Imantalab: J. Alloys Comp., 2015, vol. 632, pp. 48–52.
A. Ehsani, M. Nasrollahzadeh, M.G. Mahjani, R. Moshrefi, and H. Mostaanzadeh: J. Ind. Eng. Chem., 2014, vol. 20, pp. 4363–70.
J.J. Gray, B.S. El Dasher, and C.A. Orme: Sur. Sci., 2006, vol. 600, pp. 2488–94.
J.J. Gray, and C.A. Orme: Electrochem. Acta., 2007, vol. 52, pp. 2370–75.
H. Wu, Y. Wang, Q. Zhong, M. Sheng, H. Du, and Z. Li: J. Electroanal. Chem., 2011, vol. 663, pp. 59–66.
M.F. Naeini, M.H. Shariat, and M. Eizadjou: J. Alloys Comp., 2011, vol. 509, pp. 4696–4700.
Y. Ashworth, and D. Fairhurst: J. Electrochem. Soc., 1977, vol. 124, pp. 506–17.
M. Pérez Sánchez, M. Barrera, S. González, R.M. Souto, R.C. Salvarezza, and A.J. Arvia: Electrochim. Acta., 1990, vol. 35, pp. 1337–43.
M.M. Laz, R.M. Souto, S. González, R.C. Salvarezza, and A.J. Arvia: Electrochim. Acta., 1992, vol. 37, pp. 655–63.
K. Nakaoka, J. Ueyama, and K. Ogura: J. Electrochem. Soc., 2004, vol. 151, pp. C661–65.
K.W. Cheng, W.C. Lee, and M.S. Fan: Electrochim. Acta., 2013, vol. 87, pp. 53– 62.
Y.K. Hsu, C.H. Yu, Y.C. Chen, and Y.G. Lin: J. Power Sources., 2013, vol. 242, pp. 541–47.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 28, 2015.
Rights and permissions
About this article
Cite this article
Fattah-alhosseini, A., Imantalab, O. Passivation Behavior of Ultrafine-Grained Pure Copper Fabricated by Accumulative Roll Bonding (ARB) Process. Metall Mater Trans A 47, 572–580 (2016). https://doi.org/10.1007/s11661-015-3239-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-015-3239-z