Abstract
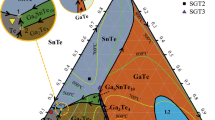
The Ag-Sn-Te ternary system is of interest to thermoelectric applications and its liquidus projection is determined in this study. Forty Ag-Sn-Te ternary alloys are prepared and their primary solidification phases are determined. These different primary solidification phase regions include three terminal solid solutions: Ag, Sn, and Te; six binary intermediate phases: SnTe, β-Ag5Te3, Ag1.9Te, Ag2Te (assuming no phase transformation), ζ-Ag4Sn, and ε-Ag3Sn; and one ternary compound, AgSnTe2. These data, together with the phase diagrams of the three constituent binary systems, are employed to construct the univariant lines of the liquidus projection. The temperature-descending directions of these univariant lines are determined using thermal analysis results and mass balance concept. The types of invariant reactions and the reaction temperatures are determined from the temperature-descending directions of the univariant lines and by thermal analysis. There are two Class I reactions, five Class II reactions, and one Class III reaction. The invariant reaction with the highest reaction temperature is L + Ag = Ag2Te + ε-Ag3Sn, at 992.7 ± 4 K (719.5 ± 4 °C), and that with the lowest reaction temperature is L = Sn + ε-Ag3Sn + SnTe, at 494.2 ± 4 K (221 ± 2 °C).















Similar content being viewed by others
References
M. Zebarjadi, K. Esfarjani, M. S. Dresselhaus, Z. F. Ren and G. Chen: Energy Environ. Sci., 2012, vol. 5, pp. 5147-5162.
H.-J. Wu, S.-W. Chen, T. Ikeda and G.J. Snyder: Acta Mater., 2012, vol. 60, pp. 1129–1138.
J. Q. Guo, H. Y. Geng, T. Ochi, S. Suzuki, M. Kikuchi, Y. Yamaguchi and S. Ito: J. Electron. Mater., 2012, vol. 41(6), pp. 1036-1042.
A. D. LaLonde, Y. Pei, H. Wang and G. J. Snyder: Mater. Today, 2011, vol. 14(11), pp. 526-532.
H.-J. Wu and S.-W. Chen: Acta Mater., 2011, vol. 59, pp. 6463–6472.
A. Shakouri: Annu. Rev. Mater. Res., 2011, vol. 41, pp. 399-431.
L. E. Bell: Science, 2008, vol. 321(5895), pp. 1457-1461.
S. M. Kauzlarich, S. R. Brown and G. J. Snyder: Dalton Trans., 2007, vol. 36(21), pp. 2099-2107.
M. S. Dresselhaus, G. Chen, M. Y. Tang, R. Yang, H. Lee, D. Wang, Z. Ren, J.-P. Fleurial and P. Gogna: Adv. Mater., 2007, vol. 19, pp. 1043-1053.
C.N. Liao and Y.C. Huang: J. Mater. Res.,2010, vol. 25,pp. 391-395.
E. I. Rogacheva: J. Phys. Chem. Solids, 2005, vol. 66(11), pp. 2104-2111.
J. Wu, J. Yang, H. Zhang, J. Zhang, S. Feng, M. Liu, J. Peng, W. Zhu, and T. Zou: J. Alloys Compd., 2010, vol. 507(1), pp. 167-171.
J. Androulakis, R. Pcionek, E. Quarez, J.-H. Do, H. Kong, O. Palchik, C. Uher, J. J. D’Angelo, J. Short, T. Hogan, and M. G. Kanatzidis: Chem. Mater., 2006, vol. 18, pp. 4719-4721.
T.P. Hogan, A.D. Downey, J. Short, J. D’Angelo, E. Quarez, J. Androulakis, P.F.P. Poudeu, M.G. Kanatzidis, E. Timm, K. Sarbo, and H. Schock: Mater. Res. Soc. Symp. Proc., J. Yang, T.P. Hogan, R. Funahashi, and G.S. Nolas, eds., 2006, vol. 886, pp. 487–92.
H. J. Liu, Y.-G. Yan, X.-F. Tang, L.-L. Yin and Q.-J. Zhang: Acta Phys., 2007, vol. 56 (12), pp. 7309-14.
V. A. Kulbachinskii, A. U. Kaminskii, P. M. Tarasov, and P. Lostak: Phys Solid State, 2006, vol. 48(5), pp. 833-840.
H. Hahn and H. Schulze: Naturwissenschaften, 1964, vol. 51(22), pp. 534.
R. Blachnik, G. Bolte, and B. Gather: Z. Metallkde., 1978, vol. 69(8), pp. 530-533.
R. Blachnik and B. Gather: J. Less Common Met., 1978, vol. 60, pp. 25-32.
G. Effenberg and B. Grieb: Ternary Alloys, VCH, New York, NY, 1988, vol. 2, pp. 590–601.
L.M. de Chalbaud, B.J. Fernandez, R. Davila, D.B. Bracho, J.M. Delgado, and A.E. Mora: Inst. Phys. Conf. Ser., 1998, vol. 152, pp. 107–110.
F. Romermann and R. Blachnik: Z. Metallkde., 2011, vol. 92(4), pp. 336-344.
F. N. Rhines, “Phase diagrams in metallurgy: Their development and application”, McGraw-Hill, New York, 1956.
O. F. Devereux, “Topics in metallurgical thermodynamics”, Krieger, Melbourne, Florida, 1983.
Y.-C. Huang, S.-W. Chen, C.-Y. Chou, W. Gierlotka: J. Alloys Compd., 2009, vol. 477(1-2), pp. 283-290.
H.-J. Wu and S.-W. Chen: J. Alloys Compd., 2011, vol. 509, pp. 656-668.
I. Karakaya and W. T. Thompson: Binary Alloy Phase Diagram, 1991, 1, pp. 94–97.
C.-S. Oh, J.-H. Shim, B.-J. Lee and D. N. Lee: J. Alloys Compd., 1996, vol. 238, pp. 155-166.
I. Karakaya and W. T. Thompson: J. Phase Equilib., 2010, vol. 12(1), pp. 56–63.
W. Gierlotka: J. Alloys Compd., 2009, vol. 485, pp. 231-235.
R. C. Sharma and Y. A. Chang: Bull. Alloy Phase Diagr., 1986, vol. 7(1), pp. 72–80.
K.-C. Hsieh, M. S. Wei and Y. A. Chang. Z. Metallkd.,1983, vol.74, pp. 330-337.
P. E. J. Flewitt and R. K. Wild: “Physical methods for materials characterization.” Institute of Physics Publishing, Bristol, 1994.
S.-W. Chen, C.-C. Huang and J.-C. Lin: Chem. Eng. Sci., 1995, vol. 50(3), pp. 417-431.
W. J. Boettinger, U. R. Kattner, K.-W. Moon and J. H. Perepezko, “DTA and heat-flux DSC measurements of alloy melting and freezing”, NIST, Washington DC, 2006.
Acknowledgments
The authors acknowledge the financial support of the National Science Council of Taiwan (NSC99-2221-E-093-MY3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 28, 2013.
Rights and permissions
About this article
Cite this article
Chang, Js., Chen, Sw., Chiu, Kc. et al. Liquidus Projection of the Ag-Sn-Te Ternary System. Metall Mater Trans A 45, 3728–3740 (2014). https://doi.org/10.1007/s11661-014-2318-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-014-2318-x