Abstract
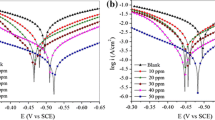
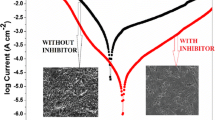
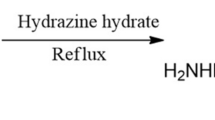
The purpose of this study was to examine the inhibitory effect of 4-amino-5-methyl-4H-1, 2, 4-triazole-3-thiol (AMTT) on the corrosion of mild steel in 1.0 M HCl solution using weight loss, electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization. The results indicate that AMTT performed as a good mixed-type inhibitor for mild steel corrosion in a 1.0 M HCl solution, and the inhibition efficiencies increased and tend to saturate with inhibitor concentration. Potentiodynamic polarization results showed that AMTT is a mixed-type inhibitor. Adsorption of AMTT molecules is a spontaneous process, and its adsorption behavior obeys Langmuir’s adsorption isotherm model. The reactivity of AMTT was analyzed through theoretical calculations based on density functional theory. Results showed that the reactive sites were located on the nitrogen and sulfur (N1, N2, and S) atoms.








Similar content being viewed by others
References
A.Y. Musa, A.A.H. Kadhum, M.S Takriff, A.R. Daud, S.K. Kamarudin, and N. Muhamad: Corros. Eng., Sci. Technol., 2010, vol. 45, pp. 163–68.
A.Y. Musa, A.A. Khadom, A.A.H. Kadhum, A.B. Mohamad, and M.S. Takriff: J. Taiwan Inst. Chem. Eng., 2010, vol. 41, pp. 126–28.
J.C. Rocha, J.A.C.P. Gomes, and E. D’Elia: Corros. Sci., 2010, vol. 52, pp. 2341–48.
J. Tang, Y. Shao, T. Zhang, G. Meng, and F. Wanga: Corros. Sci., 2011, vol. 53, pp. 1715–23.
A.Y. Musa, A.A.H. Kadhum, A.B. Mohamad, M.S. Takriff, A.R. Daud, and S.K. Kamarudin: Corros. Sci., 2009, vol. 51, pp. 2393–99.
S. Issaadi, T. Douadi, A. Zouaoui, S. Chafaa, M.A. Khan, and G. Bouet: Corros. Sci., 2011, vol. 53, pp. 1484–88.
N.A. Negm, A.M. Al Sabagh, M.A. Migahed, H.M. Abdel Bary, and H.M. El Din: Corros. Sci., 2010, vol. 52, pp. 2122–32.
P. Zhao, C. Zhong, L. Hunag, L. Niu, and F. Zhang: Corros. Sci., 2008, vol. 50, pp. 2166–71.
R. Solmaz, G. Kardas, M.C. Ulha, B. Yazıcı, and M. Erbil: Electrochimica Acta., 2008, vol. 3, pp. 5941–52.
E.E. Ebenso, U.J. Ekpe, B.I. Ita, O.E. Offiong, and U.J. Ibok: Mater. Chem. Phys., 1999, vol. 60, pp. 79–90.
G. Bereket, C. Öğretir, and Ç. Zahin: J. Mol. Struct. (THEOCHEM), 2003, vol. 663, pp. 39–46.
F.B. Growcock: Corros., 1989, vol. 45, pp. 1003–07.
F. Kandemirli and S. Sagdinc: Corros. Sci., 2007, vol. 49, pp. 2118–30.
D. Wang, S. Li, Y. Ying, M. Wang, H. Xiao, and Z. Chen: Corros. Sci., 1999, vol. 41, pp. 1911–19.
Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens, ASTM G1-3, ASTM, Philadelphia, PA, 2011, pp. 1–9, DOI: 10.1520/G0001-03R11.
L.L. Sheir, R.A. Jarman, and G.T. Burstein: Corrosion, vol. 2, Corrosion Control, chapter 11, Butterworth-Heinemann, Oxford, United Kingdom, 2000, pp. 1–55.
K.F. Khalid: J. Appl. Electro., 2010, vol. 40, pp. 601–08.
O. Benali, L. Larabi, S.M. Mekelleche, and Y. Harek: J. Mater. Sci., 2006, vol. 41, pp. 7064–68.
A.Y. Musa, A.A.H. Kadhum, A.B. Mohamad, M.S. Takriff, A.R. Daud, and S.K. Kamarudin: Corros. Sci. 2010, vol. 52, pp. 526–33.
W. Li, Q. He, S. Zhang, C. Pei, and B. Hou: J. Appl. Electrochem., 2008, vol. 38, pp. 289–96.
H. Ashassi-Sorkhabi and E. Asghari: Electrochimica Acta, 2008, vol. 54, pp. 162–67.
L. Herrag, B. Hammouti, S. Elkadiri, A. Aouniti, C. Jama, H. Vezin, and F. Bentiss: Corros. Sci., 2010, vol. 52, pp. 3042–51.
M. Behpour, S.M. Ghoreishi, N. Soltani, M. Salavati-Niasari, M. Hamadanian, and A. Gandomi: Corros. Sci., 2008, vol. 50, pp. 2172–81.
R. Solmaz: Corros. Sci., 2010, vol. 52, pp. 3321–30.
X. Li, L. Tang, H. Lui, G. Mu, and G. Lui: Mater. Lett., 2008, vol. 62, pp. 2321–24.
A.Y. Musa, A.A.H. Kadhum, A.B. Mohamad, A.A. Rahoma, and H. Mesmari: J. Molec. Struct., 2010, vol. 969, pp. 233–37.
T. Arslan, F. Kandemirli, Eno E. Ebenso, I. Love, and H. Alemu: Corros. Sci., 2009, vol. 51, pp. 35–47.
F. Kandemirli and S. Sagdinc: Corros. Sci., 2007, vol. 49, pp. 2118–30.
P. Geerlings and F. De Proft: Int. J. Mol. Sci., 2002, vol. 3, pp. 276–309.
Y. Li and J.N.S. Evans: J. Am. Chem. Soc., 1995, vol. 117, pp. 7756–59.
Acknowledgments
One of the authors (AYM) gratefully acknowledges the Universiti Kebangsaan Malaysia for the support of this project under Grant No. UKM-GGPM-NBT-037-2011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted February 13, 2011.
Rights and permissions
About this article
Cite this article
Musa, A.Y., Mohamad, A.B., Kadhum, A.A.H. et al. Corrosion Inhibition of Mild Steel in 1.0 M HCl by Amino Compound: Electrochemical and DFT Studies. Metall Mater Trans A 43, 3379–3386 (2012). https://doi.org/10.1007/s11661-012-1122-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-012-1122-8