Abstract
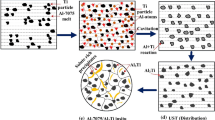
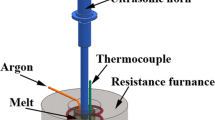
Different types of nanoparticles in aluminum (Al) alloy A356 nanocomposites were shown to catalyze nucleation of the primary Al phase. Nanoparticles of SiC β, TiC, Al2O3 α, and Al2O3 γ were added to and dispersed in the A356 matrix as nucleation catalysts using an ultrasonic mixing technique. Using the droplet emulsion technique (DET), undercoolings in the nanocomposites were shown to be significantly reduced compared to the reference A356. None of the nanocomposites had a population of highly undercooled droplets that were observed in the reference samples. Also, with the exception of the A356/Al2O3 α nanocomposite, all nanocomposites showed a reduction in undercooling necessary for the onset of primary Al nucleation. The observed nanocomposite undercoolings generally agreed with the undercooling necessary for free growth. The atomic structure of the particles showed an influence on nucleation potency as A356/Al2O3 γ nanocomposites had smaller undercoolings than A356/Al2O3 α nanocomposites. The nucleation catalysis illustrates the feasibility of, and basis for, grain refinement in metal matrix nanocomposites (MMNCs).








Similar content being viewed by others
References
T.E. Quested: Mater. Sci. Technol., 2004, vol. 20, pp. 1357–69.
S.F. Hassan and M. Gupta: Mater. Sci. Technol., 2004, vol. 20, pp. 1383–88.
S.F. Hassan and M. Gupta: J. Compos. Mater., 2007, vol. 41, pp. 2533–43.
Y.T. Zhao, S.L. Zhang, G. Chen, X.N. Cheng, and C.Q. Wang: Compos. Sci. Technol., 2008, vol. 68, pp. 1463–70.
X.C. Tong and H.S. Fang: Metall. Mater. Trans. A, 1998, vol. 29A, pp. 893–902.
A.F. Zimmerman, G. Palumbo, K.T. Aust, and U. Erb: Mater. Sci. Eng. A, 2002, vol. 328, pp. 137–46.
M.J. Tan and X. Zhang: Mater. Sci. Eng. A, 1998, vol. A244, pp. 80–85.
H. Ferkel and B.L. Mordike: Mater. Sci. Eng. A, 2001, vol. A298, pp. 193–99.
D.Y. Ying and D.L. Zhang: Mater. Sci. Eng., A, 2000, vol. 286, pp. 152–56.
M.C. Flemings: Solidification Processing, McGraw-Hill Inc., New York, NY, 1974, pp. 290–327.
W.T. Kim and B. Cantor: Acta Metall. Mater., 1994, vol. 42, pp. 3115–27.
N. Marasli and J.D. Hunt: J. Cryst. Growth, 1998, vol. 191, pp. 558–62.
W.T. Kim and B. Cantor: Acta Metall. Mater., 1992, vol. 40, pp. 3339–47.
W.T. Kim and B. Cantor: Acta Metall. Mater., 1994, vol. 42, pp. 3045–53.
B. Cantor: Philos. Trans. R. Soc. A, 2003, vol. 361, pp. 409–17.
A.L. Greer and T.E. Quested: Philos. Mag., 2006, vol. 86, pp. 3665–80.
R.C. Weast: CRC Handbook of Chemistry and Physics, 65th ed., CRC Presses Inc., Boca Raton, FL, 1984, pp. D43–D46.
T.E. Quested and A.L. Greer: Acta Mater., 2004, vol. 52, pp. 3859–68.
A.L. Greer, P.S. Cooper, M.W. Meredith, W. Schneider, P. Schumacher, J.A. Spittle, and A. Tronche: Adv. Eng. Mater., 2003, vol. 5, pp. 81–91.
A. Tronche and A.L. Greer: Philos. Mag. Lett., 2001, vol. 81, pp. 321–28.
R. Gunther, C. Hartig, and R. Bormann: Acta Mater., 2006, vol. 54, pp. 5591–97.
M. Qian: Acta Mater., 2007, vol. 55, pp. 943–53.
B.L. Bramfitt: Metall. Trans., 1970, vol. 1, pp. 1987–95.
A. Luo: Can. Metall. Q., 1996, vol. 35, pp. 375–83.
Y. Yang, J. Lan, and X. Li: Mater. Sci. Eng. A, 2004, vol. 380, pp. 378–83.
M.K. Hoffmeyer and J.H. Perepezko: Scripta Metall., 1988, vol. 22, pp. 1143–48.
M. De Cicco, L.S. Turng, X. Li, and J.H. Perepezko: Solid State Phenomenon B: Diffus. Def. Data, 2008, vols. 141–143, pp. 487–92.
P. Schumacher, A.L. Greer, J. Worth, P.V. Evans, M.A. Kearns, P. Fisher, and A.H. Green: Mater. Sci. Technol., 1998, vol. 14, pp. 394–404.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 18, 2010.
Rights and permissions
About this article
Cite this article
De Cicco, M.P., Turng, LS., Li, X. et al. Nucleation Catalysis in Aluminum Alloy A356 Using Nanoscale Inoculants. Metall Mater Trans A 42, 2323–2330 (2011). https://doi.org/10.1007/s11661-011-0607-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-011-0607-1