Abstract
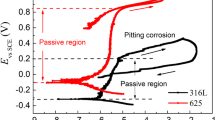
Alloy 22 (N06022) is the current candidate alloy used to fabricate the external wall of the high-level nuclear waste containers for the Yucca Mountain repository. It was of interest to study and compare the general and localized corrosion susceptibility of Alloy 22 in fluoride and chloride solutions at 90 °C. Standard electrochemical tests such as cyclic potentiodynamic polarization, amperometry, and electrochemical impedance spectroscopy were used. Studied variables included the solution pH and the alloy microstructure (thermal aging). Results show that Alloy 22 is highly resistant to general corrosion in all the solutions tested. Thermal aging is not detrimental and even seems to be slightly beneficial for general corrosion at the higher solution pHs. Pitting corrosion was never observed. Crevice corrosion was found only for high chloride-containing solutions after anodic polarization. The presence of fluoride ions together with chloride ions seems to increase the susceptibility of Alloy 22 to crevice corrosion compared to pure chloride solutions.
Similar content being viewed by others
References
Yucca Mountain Science and Engineering Report No. DOE/RW-0539, United States Department of Energy, Office of Civilian Radioactive Waste Management, Las Vegas, NV, May 2001.
Civilian Radioactive Waste Management, System Management and Operating Contractor, Civilian Radioactive Waste Management System, System Management and Operating Contract No. TDR-EBS-MD-000010, Revision 00, ICN 01, Las Vegas, NV, 2000.
Civilian Radioactive Waste Management, System Management and Operating Contractor, Civilian Radioactive Waste Management System, System Management and Operating Contract No. SDD-UDC-SE-000001, Revision 01, ICN 01, Las Vegas, NV, 2000.
R.B. Rebak and J.C. Estill: Materials Research Society Symposia Proceedings, Materials Research Society, Warrendale, PA, 2003, vol. 757, pp. 713–21.
R.B. Rebak: Proc. Symp. on Passivity and Its Breakdown, The Electrochemical Society Inc., Pennington, NJ, 1998, vol. 97 (26), pp. 1001–12.
A.K. Roy, D.L. Fleming, and B.Y. Lum: MP, 1998, Mar., pp. 54.
R.B. Rebak and N.E. Koon: Corrosion/98, NACE International, Houston, TX, 1998, paper no. 153.
M. Raghavan, B.J. Berkowitz, and J.C. Scanlon: Metall. Trans. A, 1982, vol. 13A, pp. 979–84.
H.M. Tawancy: J. Mater. Sci., 1996, vol. 31, p. 3929.
F.G. Hodge and H.S. Ahluwalia: Proc. 12th Int. Corrosion Congr., NACE International, Houston, TX, 1993, vol. 5B, p. 4031.
B.F. Brown, J. Kruger, and R.W. Staehle: Localized Corrosion, NACE International, Houston, TX, 1986.
N.S. Meck, P. Crook, S.D. Day, and R.B. Rebak: Corrosion/03, NACE International, Houston, TX, 2003, paper no. 03682.
G.H. Koch: in Halides Other Than Chlorides, MTI Publication 41, Materials Technology Institute of the Chemical Process Industries, Inc., Columbus, OH, published by NACE International, Houston, TX, 1995.
Annual Book of ASTM Standards, ASTM, West Conshohocken, PA, 2001, vol. 03.02.
M.A. Rodríguez, R.M. Carranza, and R.B. Rebak: Corrosion/04, NACE International, Houston, TX, 1998, paper no. 04700.
M. Pourbaix: Atlas of Electrochemical Equilibrium in Aqueous Solutions, 2nd English ed., NACE, Houston, TX, 1974.
S.D. Day, K.J. Evans, and G.O. Ilevbare: Proc. Int. Symp. on Critical Factors in Localized Corrosion IV, A Symposium in Honor of the 65th Birthday of Hans Böni, S. Virtanen, P. Schmuki, and G.S. Frankel, eds., The Electrochemical Society Inc., Pennington, NJ, 2003, vol. 2002 (24), pp. 535–44.
R.M. Carranza and J.R. Galvele: Corr. Sci., 1988, vol. 28, pp. 233–49.
J.C. Estill, G.A. Hust, and R.B. Rebak: Corrosion/03, NACE International, Houston, TX, 1998, paper no. 03688.
A.J. Bard and L.R. Faulkner: Electrochemical Methods. Fundamentals and Applications, 1st ed., John Wiley & Sons, New York, NY, 1980, p. 509.
M. Da Cunha Belo, N.E. Hakiki, and M.G.S. Ferreira: Electrochimica Acta, 1999, vol. 44, pp. 2473–81.
S. Boudin, J.-L. Vignes, G. Lorang, M. Da Cunha Belo, G. Blondiaux, S.M. Mikhailov, J.P. Jacobs, and H.H. Brongersma: Surface Interface Analysis, 1994, vol. 22, pp. 462–66.
Author information
Authors and Affiliations
Additional information
This article is based on a presentation made in the symposium “Effect of Processing on Materials Properties for Nuclear Waste Disposition,” November 10–11, 2003, at the TMS Fall meeting in Chicago, Illinois, under the joint auspices of the TMS Corrosion and Environmental Effects and Nuclear Materials Committees.
Rights and permissions
About this article
Cite this article
Rodríguez, M.A., Carranza, R.M. & Rebak, R.B. Influence of halide ions and alloy microstructure on the passive and localized corrosion behavior of alloy 22. Metall Mater Trans A 36, 1179–1185 (2005). https://doi.org/10.1007/s11661-005-0210-4
Issue Date:
DOI: https://doi.org/10.1007/s11661-005-0210-4