Abstract
Objective
To explore the rapid antidepressant potential and the underlying mechanism of Chaihu Shugan San (CSS) in female mice.
Methods
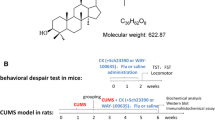
Liquid chromatography mass spectrometry (LC-MS)/MS was used to determine the content of main components in CSS to determine its stability. Female C57BL/6J mice were randomly divided into 4 groups, including control (saline), vehicle (saline), CSS (4 g/kg) and ketamine (30 mg/kg) groups. Mice were subjected to irregular stress stimulation for 4 weeks to establish the chronic mild stress (CMS) model, then received a single administration of drugs. Two hours later, the behavioral tests were performed, including open field test, tail suspension test (TST), forced swimming test (FST), novelty suppression feeding test (NSF), and sucrose preference test (SPT). Western blot analysis was used to detect the expression levels of N-methyl-D-aspartate receptor (NMDA) subtypes [N-methyl-D-aspartate receptor 1 (NR1), NR2A, NR2B], synaptic proteins [synapsin1 and post synaptic density protein 95 (PSD95)], and brain-derived neurotrophic factor (BDNF). Moreover, the rapid antidepressant effect of CSS was tested by pharmacological technologies and optogenetic interventions that activated glutamate receptors, NMDA.
Results
Compared with the vehicle group, a single administration of CSS (4 g/kg) reversed all behavioral defects in TST, FST, SPT and NSF caused by CMS (P<0.05 or P<0.01). CSS also significantly decreased the expressions of NMDA subtypes (NR1, NR2A, NR2B) at 2 h in hippocampus of mice (all P<0.01). In addition, similar to ketamine, CSS increased levels of synaptic proteins and BDNF (P<0.05 or P<0.01). Furthermore, the rapid antidepressant effects of CSS were blocked by transient activation of NMDA receptors in the hippocampus (all P<0.01).
Conclusion
Rapid antidepressant effects of CSS by improving behavioral deficits in female CMS mice depended on rapid suppression of NMDA receptors and activation of synaptic proteins.
Similar content being viewed by others
References
Ely BA, Nguyen TNB, Tobe RH, Walker AM, Gabbay V. Multimodal investigations of reward circuitry and anhedonia in adolescent depression. Front Psychiatry 2021;12:678709.
Wang Z, Cheng YT, Lu Y, Sun GQ, Pei L. Baicalin ameliorates corticosterone-induced depression by promoting neurodevelopment of hippocampal via mTOR/GSK3 β pathway. Chin J Integr Med 2023;29:405–412.
Kleih TS, Entringer S, Scholaske L, Kathmann N, DePunder K, Heim CM, et al. Exposure to childhood maltreatment and systemic inflammation across pregnancy: the moderating role of depressive symptomatology. Brain Behav Immun 2022;101:397–409.
Kanezawa S, Zhu YB, Wang Q. Correlation between Chinese medicine constitution and skin types: a study on 187 Japanese women. Chin J Integr Med 2020;26:174–179.
Wang J, Cosci F. Neonatal withdrawal syndrome following late in utero exposure to selective serotonin reuptake inhibitors: a systematic review and meta-analysis of observational studies. Psychother Psychosom 2021;90:299–307.
Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression—first FDA-approved antidepressant in a new class. N Engl J Med 2019;381:1–4.
Wilkinson ST, Sanacora G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov Today 2019;24:606–615.
McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry 2021;178:383–399.
Shi X, Zhang Q, Li J, Liu X, Zhang Y, Huang M, et al. Disrupting phosphorylation of Tyr-1070 at GluN2B selectively produces resilience to depression-like behaviors. Cell Rep 2021;36:109612.
Akil H, Nestler EJ. The neurobiology of stress: vulnerability, resilience, and major depression. Proc Natl Acad Sci U S A 2023;120:e2312662120.
Huang S, Deng Q, Zhao Y, Chen G, Geng A, Wang X. l-Glutamate seed priming enhances 2-acetyl-1-pyrroline formation in fragrant rice seedlings in response to arsenite stress. J Agric Food Chem 2023;71:18443–18453.
Natarajan S, Abass G, Kim L, Wells C, Rezvani AH, Levin ED. Acute and chronic glutamate NMDA antagonist treatment attenuates dopamine D1 antagonist-induced reduction of nicotine self-administration in female rats. Pharmacol Biochem Behav 2023;234:173678.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010;329:959–964.
Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci 2012;15:746–753.
Xia B, Zhang H, Xue W, Tao W, Chen C, Wu R, et al. Instant and lasting down-regulation of NR1 expression in the hippocampus is associated temporally with antidepressant activity after acute Yueju. Cell Mol Neurobiol 2016;36:1189–1196.
Zhang H, Sun Y, Qian S, Ge R, Guo X, Shen Q, et al. Yueju-Ganmaidazao Decoction confers rapid antidepressant-like effects and the involvement of suppression of NMDA/NO/cGMP signaling. J Ethnopharmacol 2020;250:112380.
Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife 2014;3:e03581.
Burgdorf JS, Zhang XL, Stanton PK, Moskal JR, Donello JE. Zelquistinel is an orally bioavailable novel NMDA receptor allosteric modulator that exhibits rapid and sustained antidepressant-like effects. Int J Neuropsychopharmacol 2022;25:979–991.
Camargo A, Dalmagro AP, Alte GA, Zeni ALB, Tasca CI, Rodrigues ALS. NMDA receptor-mediated modulation on glutamine synthetase and glial glutamate transporter GLT-1 is involved in the antidepressant-like and neuroprotective effects of guanosine. Chem Biol Interact 2023;375:110440.
Araujo JRC, Junior J, Damasceno M, Santos S, Vieira-Neto AE, Lobo MDP, et al. Neuropharmacological characterization of frutalin in mice: evidence of an antidepressant-like effect mediated by the NMDA receptor/NO/cGMP pathway. Int J Biol Macromol 2018;112:548–554.
Gordillo-Salas M, Pilar-Cuellar F, Auberson YP, Adell A. Signaling pathways responsible for the rapid antidepressant-like effects of a GluN2A-preferring NMDA receptor antagonist. Transl Psychiatry 2018;8:84.
Shi ZW, Kang LP, Peng HS, Yang SH, Zhang LX, Jing ZX, et al. Exploring the clinical characters of Shugan Jieyu Capsule through text mining. China J Chin Mater Med (Chin) 2017;42:3435–3442.
Xie W, Qiu X, Huang X, Xie Y, Wu K, Wang Y, et al. Comparison between the pharmacokinetics of meranzin hydrate in a rat model of chronic depression and in controls following the oral administration of Chaihu-Shugan-San. Exp Ther Med 2013;6:913–918.
Jia KK, Pan SM, Ding H, Liu JH, Zheng YJ, Wang SJ, et al. Chaihu-shugan San inhibits inflammatory response to improve insulin signaling in liver and prefrontal cortex of CUMS rats with glucose intolerance. Biomed Pharmacother 2018;103:1415–1428.
Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress 2017;6:78–93.
Weinstock M. Prenatal stressors in rodents: effects on behavior. Neurobiol Stress 2017;6:3–13.
Liu YL, Xu JJ, Han LR, Liu XF, Lin MH, Wang Y, et al. Meranzin hydrate improves depression-like behaviors and hypomotility via ghrelin and neurocircuitry. Chin J Integr Med 2023;29:490–499.
Wu L, Zhang T, Chen K, Lu C, Liu XF, Zhou JL, et al. Rapid antidepressant-like effect of Fructus Aurantii depends on cAMP-response element binding protein/brain-derived neurotrophic facto by mediating synaptic transmission. Phytother Res 2021;35:404–414.
Shi X, Bai H, Wang J, Wang J, Huang L, He M, et al. Behavioral assessment of sensory, motor, emotion, and cognition in rodent models of intracerebral hemorrhage. Front Neurol 2021;12:667511.
Camargo A, Pazini FL, Rosa JM, Wolin IAV, Moretti M, Rosa PB, et al. Augmentation effect of ketamine by guanosine in the novelty-suppressed feeding test is dependent on mTOR signaling pathway. J Psychiatr Res 2019;115:103–112.
Pesarico AP, Stangherlin EC, Rosa SG, Mantovani AC, Zeni G, Nogueira CW. Contribution of NMDA, GABAA and GABAB receptors and l-arginine-NO-cGMP, MEK1/2 and CaMK-II pathways in the antidepressant-like effect of 7-fluoro-1,3-diphenylisoquinoline-1-amine in mice. Eur J Pharmacol 2016;782:6–13.
Han SK, Kim JK, Park HS, Shin YJ, Kim DH. Chaihu-Shugan-San (Shihosogansan) alleviates restraint stress-generated anxiety and depression in mice by regulating NF-κ B-mediated BDNF expression through the modulation of gut microbiota. Chin Med 2021;16:77.
Zhang S, Lu Y, Chen W, Shi W, Zhao Q, Zhao J, et al. Network pharmacology and experimental evidence: PI3K/AKT signaling pathway is involved in the antidepressive roles of Chaihu Shugan San. Drug Des Devel Ther 2021;15:3425–3441.
Zeng Q, Li L, Siu W, Jin Y, Cao M, Li W, et al. A combined molecular biology and network pharmacology approach to investigate the multi-target mechanisms of Chaihu Shugan San on Alzheimer’s disease. Biomed Pharmacother 2019;120:109370.
Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Invest 2020;130:1336–1349.
Xue W, Zhou X, Yi N, Jiang L, Tao W, Wu R, et al. Yueju Pill rapidly induces antidepressant-like effects and acutely enhances BDNF expression in mouse brain. Evid Based Complement Alternat Med 2013;2013:184367.
Dogra S, Kumar A, Umrao D, Sahasrabuddhe AA, Yadav PN. Chronic kappa opioid receptor activation modulates NR2B: implication in treatment resistant depression. Sci Rep 2016;6:33401.
Jiang J, Zheng Y, Chen Y, Zahra A, Long C, Yang L. Exposure to prenatal antidepressant alters medial prefrontalstriatal synchronization in mice. Brain Res 2019;1717:27–34.
Murphy N, Lijffijt M, Ramakrishnan N, Vo-Le B, Vo-Le B, Iqbal S, et al. Does mismatch negativity have utility for NMDA receptor drug development in depression? Braz J Psychiatry 2022;44:61–73.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 2016;22:238–249.
Tang XH, Zhang GF, Xu N, Duan GF, Jia M, Liu R Z, et al. Extrasynaptic CaMK II α is involved in the antidepressant effects of ketamine by downregulating GluN2B receptors in an LPS-induced depression model. J Neuroinflammation 2020;17:181.
Viana GSB, Vale EMD, Araujo ARA, Coelho NC, Andrade SM, Costa ROD, et al. Rapid and long-lasting antidepressant-like effects of ketamine and their relationship with the expression of brain enzymes, BDNF, and astrocytes. Braz J Med Biol Res 2020;54:e10107.
Aleksandrova LR, Phillips AG. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci 2021;42:929–942.
Chen MH, Wu HJ, Li CT, Lin WC, Tsai SJ, Hong CJ, et al. Low-dose ketamine infusion for treating subjective cognitive, somatic, and affective depression symptoms of treatment-resistant depression. Asian J Psychiatr 2021;66:102869.
Author information
Authors and Affiliations
Contributions
Lu C, Gao ZW and Wu L conceived and designed the experiments. Wu L, Gao ZW, Huang YK, Lu C, and Wang HH performed the experiments. Wu L, Lu C and Zhou H analyzed the data. Lu C, Xing S and Wu L contributed to the writing of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
The authors declare no competing financial interest.
Additional information
Supported by the National Natural Science Foundation of China (No. 82174107, 82304898), Jiangsu Provincial Administration of Traditional Chinese Medicine Fund (No. YB2020014), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (No. 035062005001)
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Lu, C., Gao, Zw., Xing, S. et al. Rapid Antidepressant-Like Potential of Chaihu Shugan San Depends on Suppressing Glutamate Neurotransmission and Activating Synaptic Proteins in Hippocampus of Female Mice. Chin. J. Integr. Med. (2024). https://doi.org/10.1007/s11655-024-3906-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s11655-024-3906-2