Abstract
Objective
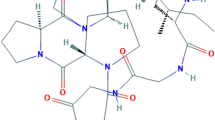
To determine whether monotropein has an anticancer effect and explore its potential mechanisms against colorectal cancer (CRC) through network pharmacology and molecular docking combined with experimental verification.
Methods
Network pharmacology and molecular docking were used to predict potential targets of monotropein against CRC. Cell counting kit assay, plate monoclonal assay and microscopic observation were used to investigate the antiproliferative effects of monotropein on CRC cells HCT116, HT29 and LoVo. Flow cytometry and scratch assay were used to analyze apoptosis and cell cycle, as well as cell migration, respectively in HCT116, HT29, and LoVo cells. Western blotting was used to detect the expression of proteins related to apoptosis, cell cycle, and cell migration, and the expression of proteins key to the Akt pathway.
Results
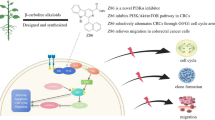
The Gene Ontology and Reactome enrichment analyses indicated that the anticancer potential of monotropein against CRC might be involved in multiple cancer-related signaling pathways. Among these pathways, RAC-beta serine/threonine-protein kinase (Akt1, Akt2), cyclin-dependent kinase 6 (CDK6), matrix metalloproteinase-9 (MMP9), epidermal growth factor receptor (EGFR), cell division control protein 42 homolog (CDC42) were shown as the potential anticancer targets of monotropein against CRC. Molecular docking suggested that monotropein may interact with the 6 targets (Akt1, Akt2, CDK6, MMP9, EGFR, CDC42). Subsequently, cell activity of HCT116, HT29 and LoVo cell lines were significantly suppressed by monotropein (P<0.05). Furthermore, our research revealed that monotropein induced cell apoptosis by inhibiting Bcl-2 and increasing Bax, induced G1–S cycle arrest in colorectal cancer by decreasing the expressions of CyclinD1, CDK4 and CDK6, inhibited cell migration by suppressing the expressions of CDC42 and MMP9 (P<0.05), and might play an anticancer role through Akt signaling pathway.
Conclusion
Monotropein exerts its antitumor effects primarily by arresting the cell cycle, causing cell apoptosis, and inhibiting cell migration. This indicates a high potential for developing novel medication for treating CRC.
Similar content being viewed by others
References
Qu R, Ma Y, Zhang Z, Fu W. Increasing burden of colorectal cancer in China. Lancet Gastroenterol Hepatol 2022;7:700.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48.
Bertelsen CA, Bols B, Ingeholm P, Jansen JE, Neuenschwander AU, Vilandt J. Can the quality of colonic surgery be improved by standardization of surgical technique with complete mesocolic excision? Colorectal Dis 2011;13:1123–1129.
Xiang Y, Guo Z, Zhu P, Chen J, Huang Y. Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Med 2019;8:1958–1975.
Zhang Z, Zhang Q, Yang H, Liu W, Zhang N, Qin L, et al. Monotropein isolated from the roots of Morinda officinalis increases osteoblastic bone formation and prevents bone loss in ovariectomized mice. Fitoterapia 2016;110:166–172.
Chen Y, Lu Y, Pei C, Liang J, Ding P, Chen S, et al. Monotropein alleviates secondary liver injury in chronic colitis by regulating TLR4/NF-kappaB signaling and NLRP3 inflammasome. Eur J Pharmacol 2020;883:173358.
Mieres-Castro D, Schmeda-Hirschmann G, Theoduloz C, Gomez-Alonso S, Perez-Navarro J, Marquez K, et al. Antioxidant activity and the isolation of polyphenols and new iridoids from Chilean Gaultheria phillyreifolia and G. poeppigii berries. Food Chem 2019;291:167–179.
Zhang Q, Zhang JH, He YQ, Zhang QL, Zhu B, Shen Y, et al. Iridoid glycosides from Morinda officinalis How. exert anti-inflammatory and anti-arthritic effects through inactivating MAPK and NF-kappaB signaling pathways. BMC Complement Med Ther 2020;20:172.
He YQ, Yang H, Shen Y, Zhang JH, Zhang ZG, Liu LL, et al. Monotropein attenuates ovariectomy and LPS-induced bone loss in mice and decreases inflammatory impairment on osteoblast through blocking activation of NF-kappaB pathway. Chem Biol Interact 2018;291:128–136.
Ezzat S, Louka ML, Zakaria ZM, Nagaty MM, Metwaly RG. Autophagy in osteoporosis: relation to oxidative stress. J Cell Biochem 2019;120:2560–2568.
Shi Y, Liu XY, Jiang YP, Zhang JB, Zhang QY, Wang NN, et al. Monotropein attenuates oxidative stress via Akt/mTOR-mediated autophagy in osteoblast cells. Biomed Pharmacother 2020;121:109566.
Zhang Y, Chen Y, Li B, Ding P, Jin D, Hou S, et al. The effect of monotropein on alleviating cisplatin-induced acute kidney injury by inhibiting oxidative damage, inflammation and apoptosis. Biomed Pharmacother 2020;129:110408.
Wang P, Kang SY, Kim SJ, Park YK, Jung HW. Monotropein improves dexamethasone-induced muscle atrophy via the AKT/mTOR/FOXO3a signaling pathways. Nutrients 2022;14:1859.
Li C, Hou SZ, Zhou H. Mechanism prediction of monotropein for the treatment of colorectal cancer by network pharmacology analysis. Digit Chin Med 2020;3:1–10.
Wang X, Shen Y, Wang S, Li S, Zhang W, Liu X, et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res 2017;45:W356–W60.
Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res 2019;47:W357–W364.
Pinero J, Ramirez-Anguita JM, Sauch-Pitarch J, Ronzano F, Centeno E, Sanz F, et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res 2020;48:D845–D855.
Xu H, Zhao H, Ding C, Jiang D, Zhao Z, Li Y, et al. Celastrol suppresses colorectal cancer via covalent targeting peroxiredoxin 1. Signal Transduct Target Ther 2023;8:51.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 2017;45:W98–W102.
Eberhardt J, Santos-Martins D, Tillack AF, Forli S. AutoDock Vina 1.2.0: new docking methods, expanded force field, and python bindings. J Chem Inf Model 2021;61:3891–3898.
Alonso H, Bliznyuk AA, Gready JE. Combining docking and molecular dynamic simulations in drug design. Med Res Rev 2006;26:531–568.
Islam MR, Akash S, Rahman MM, Nowrin FT, Akter T, Shohag S, et al. Colon cancer and colorectal cancer: Prevention and treatment by potential natural products. Chem Biol Interact 2022;368:110170.
Wolf P, Schoeniger A, Edlich F. Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane. Biochim Biophys Acta Mol Cell Res 2022;1869:119317.
Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol 2013;14:518–528.
Nebenfuehr S, Kollmann K, Sexl V. The role of CDK6 in cancer. Int J Cancer 2020;147:2988–2995.
Qadir MI, Parveen A, Ali M. Cdc42: role in cancer management. Chem Biol Drug Des 2015;86:432–439.
Wang R, Ke ZF, Wang F, Zhang WH, Wang YF, Li SH, et al. GOLPH3 overexpression is closely correlated with poor prognosis in human non-small cell lung cancer and mediates its metastasis through upregulating MMP-2 and MMP-9. Cell Physiol Biochem 2015;35:969–982.
Ahmad R, Shihab PK, Jasem S, Behbehani K. FSL-1 induces MMP-9 production through TLR-2 and NF-kappaB /AP-1 signaling pathways in monocytic THP-1 cells. Cell Physiol Biochem 2014;34:929–942.
Yang CQ, Li W, Li SQ, Li J, Li YW, Kong SX, et al. MCP-1 stimulates MMP-9 expression via ERK 1/2 and p38 MAPK signaling pathways in human aortic smooth muscle cells. Cell Physiol Biochem 2014;34:266–276.
Wang Q, Yu W, Huang T, Zhu Y, Huang C. RUNX2 promotes hepatocellular carcinoma cell migration and invasion by upregulating MMP9 expression. Oncol Rep 2016;36:2777–2784.
Li X, Ma G, Guo W, Mu N, Wang Y, Liu X, et al. Hhex inhibits cell migration via regulating RHOA/CDC42-CFL1 axis in human lung cancer cells. Cell Commun Signal 2021;19:80.
Lubbe WJ, Zhou ZY, Fu W, Zuzga D, Schulz S, Fridman R, et al. Tumor epithelial cell matrix metalloproteinase 9 is a target for antimetastatic therapy in colorectal cancer. Clin Cancer Res 2006;12:1876–1882.
Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin Cancer Biol 2022;80:1–17.
Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res 2019;79:1019–1031.
Author information
Authors and Affiliations
Contributions
Shang RY provided financial support, guided the overall idea of the article, and reviewed and edited the final manuscript; Sheng QS provided the cells, and reviewed and edited the final manuscript; Jin LJ initiated the study, developed the concept of the paper, and reviewed and edited the final manuscript; Li L completed the network pharmacology section; Gao Q completed the molecular docking and experimental part; Gao Q and Li L wrote the original manuscript; Zhang QM and Zhang JL contributed to materials information gathering and data analysis. Gao Q and Li L contribute equally to this work as co-first authors.
Corresponding authors
Ethics declarations
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Gao, Q., Li, L., Zhang, Qm. et al. Monotropein Induced Apoptosis and Suppressed Cell Cycle Progression in Colorectal Cancer Cells. Chin. J. Integr. Med. 30, 25–33 (2024). https://doi.org/10.1007/s11655-023-3710-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-023-3710-4