Abstract
Objective
To evaluate the efficacy and safety of Cidan Capsule combined with adjuvant transarterial chemoembolization (TACE) in patients with a high risk of early recurrence after curative resection of hepatocellular carcinoma (HCC).
Methods
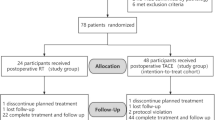
A multicenter, randomized controlled trial was conducted in patients with high-risk recurrence factors after curative resection of HCC from 9 medical centers between July 2014 and July 2018. Totally 249 patients were randomly assigned to TACE with or without Cidan Capsule administration groups by stratified block in a 1:1 ratio. Postoperative adjuvant TACE was given 4–5 weeks after hepatic resection in both groups. Additionally, 125 patients in the TACE plus Cidan group were administrated Cidan Capsule (0.27 g/capsule, 5 capsules every time, 4 times a day) for 6 months with a 24-month follow-up. Primary endpoints included disease-free survival (DFS) and tumor recurrence rate (TRR). Secondary endpoint was overall survival (OS). Any drug-related adverse events (AEs) were observed and recorded.
Results
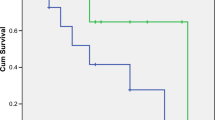
As the data cutoff in July 9th, 2018, the median DFS was not reached in the TACE plus Cidan group and 234.0 days in the TACE group (hazard ratio, 0.420, 95% confidence interval, 0.290–0.608; P<0.01). The 1- and 2-year TRR in the TACE plus Cidan and TACE groups were 31.5%, 37.1%, and 60.8%, 63.4%, respectively (P<0.01). Median OS was not reached in both groups. The 1- and 2-year OS rates in TACE plus Cidan and TACE groups were 98.4%, 98.4%, and 89.5%, 87.9%, respectively (P<0.05). The most common grade 3–4 AEs included fatigue, abdominal pain, lumbar pain, and nausea. One serious AE was reported in 1 patient in the TACE plus Cidan group, the death was due to retroperitoneal mass hemorrhage and hemorrhagic shock, and was not related to study drug.
Conclusions
Cidan Capsule in combination with TACE can reduce the incidence of early recurrence in HCC patients at high-risk of recurrence after radical hepatectomy and may be an appropriate option in postoperative anti-recurrence treatment. (Registration No. NCT 02253511)
Similar content being viewed by others
References
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:7.
Benson AB, D’Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, et al. Guidelines insights: hepatobiliary cancers, version 2. 2019. J Natl Compr Canc Netw 2019;17:302–310.
Du Y, Su T, Ding Y, Cao G. Effects of antiviral therapy on the recurrence of hepatocellular carcinoma after curative resection or liver transplantation. Hepat Mon 2012;12:e6031.
Dong ZR, Zhang PF, Wang CH, Zhang C, Cai JB, Shi GM, et al. Postoperative adjuvant transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinomas beyond the Milan criteria: a retrospective analysis. Am J Cancer Res 2014;5:450–457.
Jia F. Progress in diagnosis, treatment, and clinical research of liver cancer in China. Chin J Pract Surg (Chin) 2019;10:17.
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750.
Liu GF, Li F, Bi XJ, Zhu BH. Systematic review of clinical effects of Cidan Capsules combined with TACE in treatment against primary liver cancer. Chin J Hosp Pharm (Chin) 2016;36:1496–1500.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–655.
Zhang Y, Zhang M, Chen M, Mei J, Xu L, Guo R, et al. Association of sustained response duration with survival after conventional transarterial chemoembolization in patients with hepatocellular carcinoma. JAMA Netw Open 2018;1:e183213.
Bruix J, Takayama T, Mazzaferro V, Chau GT, Yang JM, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM) a phase 3, randomised, doubleblind, placebo-controlled trial. Lancet Oncol 2015;16:1344–1354.
Zhang XP, Wang K, Wang M, Yang G, Ye XF, Wu MC, et al. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget 2017;8:29416–29427.
Ling CQ. Experiences on the establishment of series clinical programs for the treatment of primary liver cancer by integrated traditional Chinese and Western medicine. Chin J Integr Tradit West Med (Chin) 2019;39:1166–1168.
Xi SY, Minuk GY. Role of traditional Chinese medicine in the management of patients with hepatocellular carcinoma. World J Hepatol 2018;10:799–806.
Zheng WD, Zheng DH, Zheng WH. Clinical summarization of Cidan Capsule in treating 325 cases of primary liver cancer. Shanghai J Tradit Chin Med (Chin) 2002;12:8.
Liu JY, He C, Tang Y, Liu WD, Xu Y, Li ZL, et al. A review of Cremastra appendiculata (D.Don) Makino as a traditional herbal medicine and its main components. J Ethnopharmacol 2021;279:114357.
Chen XP, Pei LX, Zhong ZF, Guo JJ, Zhang QW, Wang YT. Antitumor potential of ethanol extract of Curcuma phaeocaulis Valeton against breast cancer cells. J Phytomed 2011;18:1238–1243.
Li K, Liang YY, Wang Q, Li Y, Zhou SJ, Wei HC, et al. Brucea javanica: a review on anticancer of its pharmacological properties and clinical researches. J Phytomed 2021;86:153560.
Rao PS, Ramanadham M, Prasad MN. Anti-proliferative and cytotoxic effects of Strychnos nux-vomica root extract on human multiple myeloma cell line—RPMI 8226. Food Chem Toxicol 2009;47:283–288.
Chen XP, Guo JJ, Bao JL, Lu JJ, Wang YT. The anticancer properties of Salvia Miltiorrhiza Bunge (Danshen): a systematic review. Medl Res Rev 2014;34:768–794.
Wang JX, Zhou CF, Zheng WD. Clinical effect of Cidan Capsule on patients with primary hepatocellular carcinoma. World J Integr Tradit West Med (Chin) 2006;1:160–162.
Li N, Zheng DH, Xue J, Guo WX, Shi J, Sun JX. et al. Cidan inhibits liver cancer cell growth by reducing COX-2 and VEGF expression and cell cycle arrest. Exp Ther Med 2015;9:1709–1718.
Brown ZJ, Tsung A. Adjuvant treatment of hepatocellular carcinoma after resection. Hepat Res 2021;7:68.
Author information
Authors and Affiliations
Contributions
Wu MC was involved in the development of study protocol, study design, data review and interpretation as joint lead investigators of this clinical trial. Zheng WD was involved in the design, writing, and revision of the manuscript. Yang JM and Wu JX were responsible for the provision of patients and data acquisition. Zheng DH, Cheng SQ and Zeng JX contributed to the review of statistical tables, interpretation of data and review of the report for medical consistency against the study database. Zhang SG, Wu D, Li AJ, Fu XH, Li X, Qi FZ, Duan WH, Chen JH, Yang ZY and Liang L were the study physician advisers and contributed to data interpretation. Zheng DH and Zeng JX were co-contributed to data analysis and wrote the manuscript. All authors provided critical review of the manuscript and approved the final version for publication.
Corresponding authors
Ethics declarations
All authors declared that there are no conflicts of interest. Zheng WD did not participate in the specific research process or in the statistical analysis of the results, so it does not affect the scientific, objectivity and authority of the research results.
Additional information
Supported by National 12th Five Year “Major New Drug Creation” Science and Technology Major Project (No. 20132x091022-023)
Rights and permissions
About this article
Cite this article
Zheng, Dh., Yang, Jm., Wu, Jx. et al. Cidan Capsule in Combination with Adjuvant Transarterial Chemoembolization Reduces Recurrence Rate after Curative Resection of Hepatocellular Carcinoma: A Multicenter, Randomized Controlled Trial. Chin. J. Integr. Med. 29, 3–9 (2023). https://doi.org/10.1007/s11655-022-3537-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-022-3537-4