Abstract
Objective
To investigate the molecular mechanism underlying the anti-hepatic fibrosis activity of ethyl acetate fraction Dicliptera chinensis (L.) Juss. (EDC) in human hepatic stellate cells (HSCs) in vitro and in a carbon tetrachloride (CCl4)-induced hepatic fibrosis mouse model in vivo.
Methods
For in vitro study, HSCs were pre-treated with platelet-derived growth factor (10 ng/mL) for 2 h to ensure activation and treated with EDC for 24 h and 48 h, respectively. The effect of EDC on HSCs was assessed using cell counting kit-8 assay, EdU staining, transmission electron microscopy, immunofluorescence staining, and Western blot, respectively. For in vivo experiments, mice were intraperitoneally injected with CCl4 (2 ° L/g, adjusted to a 25% concentration in olive oil), 3 times per week for 6 weeks, to develop a hepatic fibrosis model. Forty 8-week-old male C57BL/6 mice were divided into 4 groups using a random number table (n=10), including control, model, positive control and EDC treatment groups. Mice in the EDC and colchicine groups were intragastrically administered EDC (0.5 g/kg) or colchicine (0.2 mg/kg) once per day for 6 weeks. Mice in the control and model groups received an equal volume of saline. Biochemical assays and histological examinations were used to assess liver damage. Protein expression levels of α -smooth muscle actin (α -SMA) and microtubule-associated protein light chain 3B (LC3B) were measured by Western blot.
Results
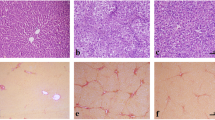
EDC reduced pathological damage associated with liver fibrosis, downregulated the expression of α -SMA and upregulated the expression of LC3B (P<0.05), both in HSCs and the CCl4-induced liver fibrosis mouse model. The intervention of bafilomycin A1 and rapamycin in HSCs strongly supported the notion that inhibition of autophagy enhanced α -SMA protein expression levels (P<0.01). The results also found that the levels of phosphoinositide (PI3K), p-PI3K, AKT, p-AKT, mammalian target of rapamycin (mTOR), p-mTOR, and p-p70S6K all decreased after EDC treatment (P<0.05).
Conclusions
EDC has anti-hepatic fibrosis activity by inducing autophagy and might be a potential drug to be further developed for human liver fibrosis therapy.
Similar content being viewed by others
References
Sun J, Xie Q, Tan D, Ning Q, Niu J, Bai X, et al. The 104-week efficacy and safety of telbivudine-based optimization strategy in chronic hepatitis B patients: a randomized, controlled study. Hepatology 2014;59:1283–1292.
Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol 2010;7:425–436.
Rockey DC. Translating an understanding of the pathogenesis of hepatic fibrosis to novel therapies. Clin Gastroenterol Hepatol 2013;11:224–231.
Ye B, Zhang Y, He W. Advances in research on Dicliptera chinensis (L.) Juss. China Pract Med (Chin) 2011;6:235–237.
Xu D, He Y, Yang S, Li K, Yang X, Shen Z. Protective effect of Dicliptera chinensis (L.) Juss. Polysaccharide P2B on carbon tetrachloride-induced injury of liver cell line L-02. China Pharm (Chin) 2016;19:675–677.
Xu Y, Gao Y, Zhong M, Li J, Cao H, Huang S, et al. Isolation, characterization and bioactivities of the polysaccharides from Dicliptera chinensis (L.) Juss. Int J Biol Macromol 2017;101:603–611.
Xu Y, Zhang K, Zhong M, Gao Y, Duan X. Advances in research on chemical constituents and pharmacology of Dicliptera chinensis (L.) Juss. Chin Pharm (Chin) 2015;34:4862–4864.
Zhang K, Zhu H, Gao Y. Research on active extracts of Dicliptera chinensis (L.) Juss. on liver protection. China J Chin Mater Med (Chin) 2010;35:497–498.
Zhang K, Gao Y, Zhong M, Xu Y, Li J, Chen Y, et al. Hepatoprotective effects of Dicliptera chinensis polysaccharides on dimethylnitrosamine-induced hepatic fibrosis rats and its underlying mechanism. J Ethnopharmacol 2016;179:38–44.
Zhang X, Zhang J, Jia L, Xiao S. Dicliptera Chinensis polysaccharides target TGF-beta/Smad pathway and inhibit stellate cells activation in rats with dimethylnitrosamine-induced hepatic fibrosis. Cell Mol Biol (Noisy-le-grand) 2016;62:99–103.
Denton D, Nicolson S, Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ 2012;19:87–95.
Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008;451:1069–1075.
Jiang P, Mizushima N. Autophagy and human diseases. Cell Res 2014;24:69–79.
San-Miguel B, Crespo I, Sánchez DI, González-Fernández B, Ortiz de Urbina JJ, Tuñón MJ, et al. Melatonin inhibits autophagy and endoplasmic reticulum stress in mice with carbon tetrachloride-induced fibrosis. J Pineal Res 2015;59:151–162.
Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut 2015;64:830–841.
Liu Y, Bi Y, Mo C, Zeng T, Huang S, Gao L, et al. Betulinic acid attenuates liver fibrosis by inducing autophagy via the mitogen-activated protein kinase/extracellular signalregulated kinase pathway. J Nat Med 2019;73:179–189.
Zhong W, Gao L, Zhou Z, Lin H, Chen C, Huang P, et al. Indoleamine 2,3-dioxygenase 1 deficiency attenuates CCl4-induced fibrosis through Th17 cells down-regulation and tryptophan 2,3-dioxygenase compensation. Oncotarget 2017;8:40486–40500.
Mimche PN, Brady LM, Bray CF, Lee CM, Thapa M, King TP, et al. The receptor tyrosine kinase EphB2 promotes hepatic fibrosis in mice. Hepatology 2015;62:900–914.
Mo C, Xie S, Zhong W, Zeng T, Huang S, Lai Y, et al. Mutual antagonism between indoleamine 2,3-dioxygenase 1 and nuclear factor E2-related factor 2 regulates the maturation status of DCs in liver fibrosis. Free Radic Biol Med 2020;160:178–190.
Dong S, Chen Q, Song Y, Sun Y, Wei B, Li X, et al. Mechanisms of CCl4-induced liver fibrosis with combined transcriptomic and proteomic analysis. J Toxicol Sci 2016;41:561–572.
Török NJ. Recent advances in the pathogenesis and diagnosis of liver fibrosis. J Gastroenterol 2008;43:315–321.
Zhao C, She T, Wang L, Su Y, Qu L, Gao Y, et al. Daucosterol inhibits cancer cell proliferation by inducing autophagy through reactive oxygen species-dependent manner. Life Sci 2015;137:37–43.
Shafik AN, Khodeir MM, Gouda NA, Mahmoud ME. Improved antifibrotic effect of a combination of verapamil and silymarin in rat-induced liver fibrosis. Arab J Gastroenterol 2011;12:143–149.
Chen SR, Chen XP, Lu JJ, Wang Y, Wang YT. Potent natural products and herbal medicines for treating liver fibrosis. Chin Med 2015;10:7.
Cheng Y, Mai JY, Wang MF, Chen GF, Ping J. Antifibrotic effect of total flavonoids of Astmgali Radix on dimethylnitrosamine-induced liver cirrhosis in rats. Chin J Integr Med 2017;23:48–54.
Zhang K, Xu Q, Gao Y, Cao H, Lian Y, Li Z, et al. Polysaccharides from Dicliptera chinensis ameliorate liver disturbance by regulating TLR-4/NF- κ B and AMPK/Nrf2 signalling pathways. J Cell Mol Med 2020;24:6397–6409.
Chen JW, Ni BB, Li B, Yang YH, Jiang SD, Jiang LS. The responses of autophagy and apoptosis to oxidative stress in nucleus pulposus cells: implications for disc degeneration. Cell Physiol Biochem 2014;34:1175–1189.
Schneider JL, Cuervo AM. Liver autophagy: much more than just taking out the trash. Nat Rev Gastroenterol Hepatol 2014;11:187–200.
Boyle KB, Randow F. The role of ‘eat-me’ signals and autophagy cargo receptors in innate immunity. Curr Opin Microbiol 2013;16:339–348.
Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 2005;1:84–91.
El-Mihi KA, Kenawy HI, El-Karef A, Elsherbiny NM, Eissa LA. Naringin attenuates thioacetamide-induced liver fibrosis in rats through modulation of the PI3K/Akt pathway. Life Sci 2017;187:50–57.
Li J, Li X, Xu W, Wang S, Hu Z, Zhang Q, et al. Antifibrotic effects of luteolin on hepatic stellate cells and liver fibrosis by targeting AKT/mTOR/p70S6K and TGFbeta/Smad signalling pathways. Liver Int 2015;35:1222–1233.
Lin X, Li Y, Zhang X, Wei Y, Wen S, Lu Z, et al. Tormentic acid inhibits hepatic stellate cells activation via blocking PI3K/Akt/mTOR and NF- κ B signalling pathways. Cell Biochem Funct 2020; doi: https://doi.org/10.1002/cbf.3564.
Fan S, Zhang B, Luan P, Gu B, Wan Q, Huang X, et al. PI3K/AKT/mTOR/p70S6K pathway is involved in A β25–35-induced autophagy. BioMed Res Int 2015;2015:161020.
Shinji S, Yukiko S, Yoko I, Sumihiro K, Takahiro F, Isei T, et al. Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy 2011;7:176–187.
Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol 2012;4:a11189.
Klionsky DJ, Meijer AJ, Codogno P. Autophagy and p70S6 kinase. Autophagy 2005;1:59–60.
Destefano MA, Jacinto E. Regulation of insulin receptor substrate-1 by mTORC2 (mammalian target of rapamycin complex 2). Biochem Soc Trans 2013;41:896–901.
Lin NY, Beyer C, Giessl A, Kireva T, Scholtysek C, Uderhardt S, et al. Autophagy regulates TNF alpha-mediated joint destruction in experimental arthritis. Ann Rheumat Dis 2013;72:761–768.
Hernández-Gea V, Hilscher M, Rozenfeld R, Lim MP, Nieto N, Werner S, et al. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J Hepatol 2013;59:98–104.
Lin CW, Zhang H, Li M, Xiong X, Chen X, Chen X, et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol 2013;58:993–999.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (No. 81673774) and the Administration of Traditional Chinese Medicine of Shandong Province (2019-0447)
Conflict of Interest
The authors declare that there is no conflict of interest.
Author Contributions
Lyu ZP, Gao L and Sun B participated in conception and design of the research. Liu Y and Bi YM carried out the experiments. Liu Y and Pan T drafted the manuscript. Zeng T participated in revision of the manuscript. Mo C participated in statistical analysis. All authors approved the final manuscript for publication.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Liu, Y., Bi, Ym., Pan, T. et al. Ethyl Acetate Fraction of Dicliptera chinensis (L.) Juss. Ameliorates Liver Fibrosis by Inducing Autophagy via PI3K/AKT/mTOR/p70S6K Signaling Pathway. Chin. J. Integr. Med. 28, 60–68 (2022). https://doi.org/10.1007/s11655-021-3298-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-021-3298-5