Abstract
Objective
To evaluate the effects of a 48-week course of adefovir dipivoxil (ADV) plus Chinese medicine (CM) therapy, namely Tiaogan Jianpi Hexue (调肝健脾和血) and Tiaogan Jiedu Huashi (调肝解毒化湿) fomulae, in hepatitis B e antigen (HBeAg)-positive Chinese patients.
Methods
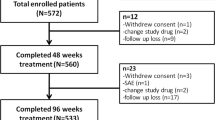
A total of 605 HBeAg-positive Chinese CHB patients were screened and 590 eligible participants were randomly assigned to 2 groups in 1:1 ratio including experimental group (EG, received ADV plus CM) and control group (CG, received ADV plus CM-placebo) for 48 weeks. The major study outcomes were the rates of HBeAg and HBV-DNA loss on week 12, 24, 36, 48, respectively. Secondary endpoints including liver functions (enzymes and bilirubin readings) were evaluated every 4 weeks at the beginning of week 24, 36, and 48. Routine blood, urine, and stool analyses in addition to electrocardiogram and abdominal B scan were monitored as safety evaluations. Adverse events (AEs) were documented.
Results
The combination therapy demonstrated superior HBeAg loss at 48 weeks, without additional AEs. The full analysis population was 560 and 280 in each group. In the EG, population achieved HBeAg loss on week 12, 24, 36, and 48 were 25 (8.90%), 34 (12.14%), 52 (18.57%), and 83 (29.64%), respectively; the equivalent numbers in the CG were 20 (7.14%), 41 (14.64%), 54 (19.29%), and 50 (17.86%), respectively. There was a statistically significant difference between these group values on week 48 (P<0.01). No additional AEs were found in EG. Subgroup analysis suggested different outcomes among treatment patterns.
Conclusion
Combination of CM and ADV therapy demonstrated superior HBeAg clearance compared with ADV monotherapy. The finding indicates that this combination therapy may provide an improved therapeutic effect and safety profile (ChiCTR-TRC-11001263).
Similar content being viewed by others
References
Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012;30:2212–2219.
Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Epidemiological serosurvey of hepatitis B in China-declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009;27:6550–6557.
Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J Infect Dis 2009;200:39–47.
Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. 2007;45:307–313.
Wu D, Wang P, Han M, Chen Y, Chen XY, Xia Q, et al. Efficacy and safety of combination therapy with interferon and immunomodulators in entecavir-suppressed chronic hepatitis B patients (the endeavor study). J Hepatol 2018;68:S509.
Ning Q, Han M, Sun Y, Jiang J, Tan D, Hou J, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised openlabel trial (OSST trial). J Hepatol 2014;61:777–784.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Intern 2016;10:1–98.
Liaw YF. HBeAg seroconversion as an important end point in the treatment of chronic hepatitis B. Hepatol Intern 2009;3:425–433.
Liaw YF, Lin DY, Chen TJ, Chu CM. Natural course after the development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Liver 1989;9:235–241.
Lin SM, Yu ML, Lee CM, Chien RN, Sheen IS, Chu CM, et al. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol 2007;46:45–52.
Liaw YF, Sung JJY, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic Hepatitis B and advanced liver disease. New Engl J Med 2004;351:1521–1531.
Chu CM, Liaw YF. Predictive factors for reactivation of hepatitis B following hepatitis B e antigen seroconversion in chronic hepatitis B. Gastroenterology 2007;133:1458–1465.
Xu WS, Zhao KK, Miao XH, Ni W, Cai X, Zhang RQ, et al. Effect of oxymatrine on the replication cycle of hepatitis B virus in vitro. World J Gastroenterol 2010;16:2028–2037.
Xia Y, Luo H, Liu JP, Gluud C. Phyllanthus species versus antiviral drugs for chronic hepatitis B virus infection. Cochrane Database Syst Rev 2013:CD009004.
Brook MG. Effect of Phyllanthus amarus on chronic carriers of hepatitis B virus. Lancet 1988;2:1017–1018.
Yeh LLL, Liu JY, Lin KS, Liu YS, Chiou JM, Liang KY, et al. A randomised placebo-controlled trial of a traditional Chinese herbal formula in the treatment of primary dysmenorrhoea. PLos One 2007;2:e719.
Kim JB, Shin JW, Kang JY, Son CG, Kang W, Lee HW, et al. A traditional herbal formula, Hyangsa-Pyeongwi San (HPS), improves quality of life (QoL) of the patient with functional dyspepsia (FD): randomized double-blinded controlled trial. J Ethnopharmacol 2014;151:279–286.
Ye YA, Tian DL, Jiang J, Li J, Chen JJ, Li ZH, et al. Effect of Shuanghu Qinggan Granule and Yigan Yiqi Jieyu Granule plus lamivudine on chronic hepatitis B patients: a randomized double-blind placebo-controlled trial. Chin J Integr Med 2016 [Epub ahead of print].
Chinese Society of Hepatology and Chinese Society of Infectious Disease CMA. The guideline of prevention and treatment for chronic hepatitis B. Chin J Hepatol (Chin) 2005;13:881–891.
Hepatology Association of WFCMS. The TCM syndrome diagnostic criteria for Chronic hepatitis B (ALT 2× ULN). J Tradit Chin Med (Chin) 2015;56:89–90.
Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology 2002;35:1522–1527.
Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med 2004;116:829–834.
Seto WK, Hui AJ, Wong VW, Wong GL, Liu KS, Lai CL, et al. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: a multicentre prospective study. Gut 2015;64:667–672.
Chen WL, Luo M, Chen XP, Huang J, Chen R. The same disease with different syndromes: a proteomic study of chronic hepatitis B. J South Med Univ (Chin) 2016;36:410–413.
Zhan T, Wei X, Chen ZQ, Wang DS, Dai XP. A systematic review of RCTs and quasi-RCTs on traditional Chinese patent medicines for treatment of chronic hepatitis B. J Tradit Chin Med 2011;31:288–296.
Xue Y, Li X, X D, Li X, Wang W, Yang J, et al. Isolation and anti-hepatitis B virus activity of dibenzocyclooctadiene lignans from the fruits of Schisandra chinensis. Phytochemistry 2015;116:253–261.
Zhao Y, Wang YJ, Zhao RZ, Xiang FJ. Vinegar amount in the process affected the components of vinegar-baked Radix Bupleuri and its hepatoprotective effect. BMC Complement Altern Med 2016;16:346.
Xing J, Sun HM, Jia JP, Qin XM, Li ZY. Integrative hepatoprotective efficacy comparison of raw and vinegar-baked Radix Bupleuri using nuclear magnetic resonance-based metabolomics. J Pharm Biomed Anal 2017;138:215–222.
Mao RC, Yin YK, Qin YL, Wu XH, Weng XH, Zhang JM, et al. Spontaneous HBeAg seroconversion and loss of hepatitis B virus DNA after acute flare due to development of drug resistant mutants during entecavir monotherapy. Hepatol Res 2009;39:14–20.
Liaw YF. Hepatitis flares and hepatitis B e antigen seroconversion: implication in anti-hepatitis B virus therapy. J Gastroenterol Hepatol 2003;18:246–252.
Dusheiko G. Treatment of HBeAg positive chronic hepatitis B: interferon or nucleoside analogues. Liver Int 2013;33 Suppl 1:137–150.
Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–283.
Acknowledgement
We thank the staff from Epidemic Research Center of Peking University Third Hospital, who conducted the statistical analysis. The online therapeutic data organization system was hosted by Jiangsu Provincial Hospital.
Author information
Authors and Affiliations
Contributions
Ye YA was the project leader and designed the protocol. Li XK, Li Y, Zhang P, Li ZG and Li S drafted the manuscript. Liu MY, Zhang KK, Yang XZ, and Gan DN helped to organize and conduct the study at Dongzhimen Hospital. Zhang MX, Zhou DQ, Shao FZ, Xue JD, Chi XL, Liu TJ, Wang XB, Lu BJ, Li J, Li Q, Yang HS, Ma DW, Yang HZ, Zhao WX, Li Y and Zhang GL were principal investigators at their respective sub-centers. Zhao YM supervised the statistical analysis and Zou JD supervised the online data service.
Corresponding author
Ethics declarations
All authors declare that they have no conflicts of interest. The sponsor of the study was not involved in the conduction, interpretation or preparation of the final manuscript.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, Xk., Zhang, Mx., Shao, Fz. et al. Adefovir Dipivoxil plus Chinese Medicine in HBeAg-Positive Chronic Hepatitis B Patients: A Randomized Controlled 48-Week Trial. Chin. J. Integr. Med. 26, 330–338 (2020). https://doi.org/10.1007/s11655-020-3250-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-020-3250-0