Abstract
Objective
To evaluate the efficacy and safety of a Chinese medicine (CM) Modified Qufeng Runmian Powder (加减祛风润面散, MQFRMP) for the treatment of acne vulgaris with CM syndromes of dampness and blood stasis.
Methods
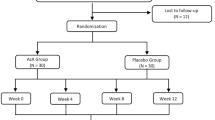
In this multicenter, randomized, double-blind, placebo-controlled clinical trial, 220 acne vulgaris patients with CM syndrome of dampness and blood stasis were included and randomly assigned using a central area group random design to receive either MQFRMP or the placebo, with 110 cases in each group. MQFRMP or a placebo at 145 g/bag were administered once daily for 4 weeks, respectively. The primary index of efficacy was the effective rate according to the acne severity score (ASS). The secondary indices of efficacy included the changes in the dermatology life quality index (DLQI) score, VISIA scores (spots, pores, brown spots, porphyrins and red areas) and skin assessment (skin pH, sebum amount and hydration) according to a SOFT skin multianalyzer.
Results
(1) Follow-up: a total of 204 patients completed the follow-up, with 103 in the treatment group and 101 in the control group. (2) Effective rate: the total effective rate of the treatment group was significantly higher than the control group [83.5% (86/103) vs. 31.7% (32/101), P<0.01)] with 95% confidence interval of 39.3%–66.4%. (3) DLQI: DLQI scores were significantly decreased the treatment and control groups (both P<0.01), but the treatment group was more obvious than the placebo group (P<0.01). (4) VISIA scores: the scores of spots, brown spots and red areas in the treatment group decreased compared with baseline (P<0.05). In the control group, the scores of brown spots and pores decreased compared with baseline (P<0.05). The improvement was more obvious in the treatment group than in the control group for all items (P<0.05). (5) Skin assessment: the pH and sebum score in the both groups decreased drastically compared with the baseline (all P<0.01), however, the improvement was more obvious in the treatment group than in the control group (P<0.01). The hydration amount in the two groups showed no statistically significant difference compared with the baseline (both P>0.05). (6) Safety: two cases of mild drug allergy were observed in the treatment group.
Conclusion
MQFRMP was effective and safe for the treatment of acne vulgaris with syndromes of dampness and blood stasis. (No. ChiCTR1900020479).
Similar content being viewed by others
References
Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet 2012;28:314.
Zhao B, ed. China clinical dermatology. 2nd ed. Jiangsu: Phoenix Science and Technology Press; 2017:1288.
Isabel L. Diagnosis and management of acne vulgaris. Nurse Prescr 2014;12:330–336.
Gollnick HP. From new findings in acne pathogenesis to new approaches in treatment. J Eur Acad Dermatol Venereol 2015;29:1–7.
Fox L, Csongradi C, Aucamp M, du Plessis J, Gerber M. Treatment modalities for acne. Molecules 2016;13:1063–1064.
Li WF, Jiang JL. Research status of pharmacological effects of curcumin. Chin J Clin Pharmacol (Chin) 2017;33:957–960.
Xu S, Jiang WQ. Research advances on chemical constituents and bioactivities of Poria cocos (Schw.) Wolf. Northwest Pharm J (Chin) 2016;31:327–330.
Wu DH. Studies on the chemical constituents of Rhizoma Kaempferiae [dissertation]. Wuhan: Huazhong University of Science and Technology;2016:16–50.
Li JF, Sun JM. Bombyx Batryticatus’s chemical components and pharmacological activities. Jilin J Tradit Chin Med (Chin) 2015;35:175–177.
Chang XD, Dai S, Ye ZZ, Song QH. Treatment of facial acne vulgaris by Chinese medicine combined Western medicine. Chin J Integr Tradit West Med (Chin) 2017;37:160–164.
Wan Y. Study on pus-draining and bloodletting with sharp-hook needle in repairing skin lesions in acne vulgaris of nodules. Shanghai J Acu-mox (Chin) 2015; 34:1204–1207.
Chen JX, ed. Diagnostics of traditional Chinese medicine. 9th ed. Beijing: People’s Medical Publishing House; 2012:130–131,152.
Qu X, ed. Dermatology and venereology of traditional Chinese medicine. 1st ed. Beijing: China Traditional Chinese Medicine Publishing House;2009:225–256.
Li YQ, ed. Chinese traditional surgery. 9th ed. Beijing: China Traditional Chinese Medicine Publishing House;2012:196–197.
Zhang XJ, Lu HG, Gao XH, eds. Dermatovenereology. 8th ed. Beijing: People’s Medical Publishing House; 2013:176.
Zheng XY, ed. Guiding principles of clinical research on new drugs of traditional Chinese medicine. 1st ed. Beijing: China Medical Science and Technology Publishing House;2002:299.
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210–216.
Goldsberry A, Hanke CW, Hanke KE. VISIA system: a possible tool in the cosmetic practice. J Drugs Dermatol 2014;13:1312–1314.
Zeng X, Liu WL, Zhao T, Zhao JY, Zhai X, Xu ZL, et al. Effects of Chinese medical facial mask comprehensive therapy in treating acne vulgaris. Chin J Integr Tradit West Med (Chin) 2012;32:624–627.
Yan WJ, Zhang L, Li N. Clinical observation on acne treated by traditional Chinese medicine combined with fire acupuncture. Inner Mongolia J Tradit Chin Med (Chin) 2018;37:6–7.
Du FX, Zhang XH, Du XZ. Clinical observation of Liangxie acupuncture in the treatment of acne. Chin J Tradit Med Sci Technol (Chin) 2018;25:276–278.
Zhou Z, Cheng J, Ouyang YL, Tian KT, Jia YB. Clinical observation on acne treated by acupuncture and cupping. Chin Manipul Rehabil Med (Chin) 2017;8:29–31.
Gui Y, Yuan Q. Clinical observation of Yinqiao Powder combined with external washing of traditional Chinese medicine in the treatment of acne. Guangming Tradit Chin Med (Chin) 2017;32:2371–2372.
Xiao JQ, Feng DP, Wang YH, Liu Q, Wu XM, Xiao J, et al. Research progress of external application of traditional Chinese medicine in the treatment of acne vulgaris. New J Tradit Chin Med (Chin) 2017;49:146–148.
Jia HK. Observation on the curative effect of Coptis chinensis Franch combined with Sanguisorba officinalis L powder for external use in the treatment of acne vulgaris. Guid China Med (Chin) 2012;10:293–294.
Wu ZX, Wang WQ, Zeng XY, Chen JB, Fan L. Exterior usage of Chinese medicine can lighten the adverse event in the clinical application of photodynamic application in the treatment of acne. J Pract Dermatol (Chin) 2015;8:129–132.
Zhang Q, Shi HQ, Zhou L, Li YH. Clinical observation on acne vulgaris treated with Acne No.1 Powder. Inner Mongolia J Tradit Chin Med (Chin) 2017;36:43–44.
Zhang YW, Hua H, Tao SQ. Observation on the curative effect of external application of traditional Chinese medicine at Yongquan point on acne vulgaris. Hebei J Tradit Chin Med (Chin) 2017;39:234–237.
Wu M, Zhang HY, Liu TF, Sun HB, Wang JF, Cao Y, et al. Clinical effect of Jiawei Yiyi Fuzi Baijiang Powder in treatment of acne with intermingled phlegm and blood stasis. J Anhui Tradit Chin Med (Chin) 2016;35:41–43.
Lin J. Therapeutic effect of traditional Chinese medicine on 32 cases of acne with phlegm and blood stasis. Chin J Etho Med Ethnopharm (Chin) 2010;19:123–124.
Author information
Authors and Affiliations
Contributions
Bai YP and Zhang TB contributed to the conception and development of the study protocol. Bai YP, Zhang TB, and Cao RQ contributed to the manuscript. Zhang TB, Cao RQ, Liu YC, Yang HY, Wu ZH, and Liu JL carried out the trial. Zhang TB, Yang HY, and Wu ZH contributed to the database management and statistical analysis. Zhang TB takes responsibility for the integrity of the work as a whole, from inception to publication. All authors have read and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Supplementary material
11655_2020_3214_MOESM1_ESM.pdf
Efficacy of Modified Qufeng Runmian Powder (加减祛风润面散) on Acne Vulgaris with Syndromes of Dampness and Blood Stasis: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Rights and permissions
About this article
Cite this article
Zhang, Tb., Bai, Yp., Yang, Hy. et al. Efficacy of Modified Qufeng Runmian Powder (加减祛风润面散) on Acne Vulgaris with Syndromes of Dampness and Blood Stasis: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Chin. J. Integr. Med. 26, 490–496 (2020). https://doi.org/10.1007/s11655-020-3214-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-020-3214-4