Abstract
Objective
To explore the specific molecular mechanisms of Danshensu (DSS) in the treatment of ischemia reperfusion injury (IRI).
Methods
IRI model was established with isolated rat hearts by performing global ischaemia for 30 min, and then followed by 60 min reperfusion. Also, H9C2 cells were subjected to 4-h hypoxia followed by 3-h reoxygenation. Then 10 μmol/L DSS were added in the reperfusion/reoxygenation step to intervene IRI. Cardiac function, structural change and apoptosis were respectively tested by Langendorff System, hematoxylin and eosin (HE) and terminal-deoxynucleotidyl transferase mediated nick endabeling (TUNEL) stainings. Then lactate dehydrogenase (LDH), reactive oxygen species (ROS), superoxide gasification enzyme (SOD) and glutathione peroxidase (GSH-PX) were detected by enzyme-linked immunosorbent assay (ELISA). Sirt1/FoxO1/Rab7 Signal Pathway was monitored at both protein and mRNA levels.
Results
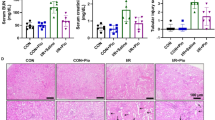
The results showed that IRI not only greatly attenuated cardiac function (LVDP and ±dp/dtmax, P<0.01, P<0.05) and increased the level of the marker enzymes (cardiac troponin T, LDH, P<0.01) from the coronary effluents, but also markedly induced changes in the structure of cardiomyocytes and contributed to apoptosis, which were mediated by boosted endogenous ROS. However, after treatment with DSS all above indexes were improved, which was related to activating Sirt1/FoxO1/Rab7 signal pathway accompanied with the enhancement of antioxidant defense system, such as superoxide gasification enzyme and glutathione peroxidase.
Conclusion
DSS is able to protect hearts from IRI, which may be attributable to inhibiting excessive ROS through Sirt1/FoxO1/Rab7 signaling.
Similar content being viewed by others
References
Cheng J, Wu Q, Lv R, Huang L, Xu B, Wang X, et al. Microrna-449a inhibition protects H9C2 cells against hypoxia/reoxygenation-induced injury by targeting the notch-1 signaling pathway. Cell Physiol Biochem 2018;46:2587–600.
Khan A, Kapoor A, Chen J, Martin L, Rogazzo M, Mercier T, et al. The antimalarial drug artesunate attenuates cardiac injury in a rodent model of myocardial infarction Shock 2018;49: 675–681.
Ghiasi R, Mohammadi M, Majidinia M, Yousefi B, Ghiasi A, Badalzadeh R. The effects of mebudipine on myocardial arrhythmia induced by ischemia-reperfusion injury in isolated rat heart. Cell Mol Biol 2016;62:15–20.
Nuntaphum W, Pongkan W, Wongjaikam S, Thummasorn S, Tanajak P, Khamseekaew J, et al. Vagus nerve stimulation exerts cardioprotection against myocardial ischemia/reperfusion injury predominantly through its efferent vagal fibers. Basic Res Cardiol 2018;113:22.
Li J, Li RJ, Lv GY, Liu HQ. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci 2015;19:2036–2047.
Kingma JG. Inhibition of Na+/H+ Exchanger with EMD 87580 does not confer greater cardioprotection beyond preconditioning on ischemia-reperfusion injury in normal dogs. Cardiovasc Pharmacol Ther 2018;23:254–69.
Huang Z, Ye B, Wang Z, Han J, Lin L, Shan P, et al. Inhibition of lncRNA-HRIM increases cell viability by regulating autophagy levels during hypoxia/reoxygenation in myocytes. Cell Physiol Biochem 2018;46:1341–1351.
Hentia C, Rizzato A, Camporesi E, Yang Z. An overview of protective strategies against ischemia/reperfusion injury: The role of hyperbaric oxygen preconditioning. Brain Behav 2018;8:e00959.
Lin B, Xu J, Feng DG, Wang F, Wang JX, Zhao H. DUSP14 knockout accelerates cardiac ischemia reperfusion (IR) injury through activating NF-kappaB and MAPKs signaling pathways modulated by ROS generation. Biochem Biophys Res Commun 2018;501:24–32.
Tanno S, Yamamoto K, Kurata Y, Adachi M, Inoue Y, Otani N, et al. Protective effects of topiroxostat on an ischemiareperfusion model of rat hearts. Circ J 2018;82:1101–1111.
Ribeiro Junior RF, Dabkowski ER, Shekar KC, O Connell KA, Hecker PA, Murphy MP. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic Biol Med 2018;117:18–29.
Wang H, Chen Y, Zhai N, Chen X, Gan F, Li H, et al. Ochratoxin A-induced apoptosis of IPEC-J2 cells through ROS-mediated mitochondrial permeability transition pore Opening Pathway. Agric Food Chem 2017;65:10630–10637.
Yamamoto T, Sadoshima J. Protection of the heart against ischemia/reperfusion by silent information regulator 1. Trends Cardiovasc Med 2011;21:27–32.
Xu H, Liu W, Liu T, Su N, Guo C, Feng X, et al. Synergistic neuroprotective effects of Danshensu and hydroxysafflor yellow A on cerebral ischemia-reperfusion injury in rats. Oncotarget 2017;8:115434–115443.
Li ZM, Xu SW, Liu PQ. Salvia miltiorrhiza Burge (Danshen): a golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol Sin 2018;39:802–824.
Yu J, Wang L, Akinyi M, Li Y, Duan Z, Zhu Y, et al. Danshensu protects isolated heart against ischemia reperfusion injury through activation of Akt/ERK1/2/Nrf2 signaling. Int J Clin Exp Med 2015;8:14793–14804.
Fan G, Yu J, Asare PF, Wang L, Zhang H, Zhang B, et al. Danshensu alleviates cardiac ischaemia/reperfusion injury by inhibiting autophagy and apoptosis via activation of mTOR signalling. Cell Mol Med 2016;20:1908–1919.
Dar MH, Adnan Y, Faheem M, Khan I, Noor L. Short term clinical outcomes of Everolimus-eluting stents in patients with stable angina pectoris. Pak J Med Sci 2018;34:235–239.
Rahmani R, Jiriaee L, Jiriaee Z, Shafiee A, Zand Parsa AF. The incidence of myocardial injury after loading doses of clopidogrel versus prasugrel in the candidates for percutaneous coronary intervention: a randomized controlled trial. Crit Pathw Cardiol 2018;17:69–72.
Gao Q, Zhao J, Fan Z, Bao J, Sun D, Li H, et al. Cardioprotective effect of Danshensu against ischemic/reperfusion injury via c-subunit of ATP synthase inhibition. Evid Based Complement Alternat Med 2017;2017:7986184.
Zhang X, Luo J, Huang Y, Deng H, Hu H, Yu P, et al. A Novel Danshensu-tetramethylpyrazine conjugate DT-018 provides cardioprotection by preserving mitochondrial function through the MEF2D/PGC-1alpha pathway. Curr Pharm Des 2018;23:6062–6070.
Lu H, Tian A, Wu J, Yang C, Xing R, Jia P, et al. Danshensu inhibits beta-adrenergic receptors-mediated cardiac fibrosis by ROS/p38 MAPK axis. Biol Pharm Bull 2014;37:961–967.
Du L, Miao X, Jiang Y, Jia H, Tian Q, Shen J, et al. An effective strategy for the synthesis of biocompatible gold nanoparticles using Danshensu antioxidant: prevention of cytotoxicity via attenuation of free radical formation. Nanotoxicology 2013;7:294–300.
Gao L, Zhao Y, He J, Yan Y, Xu L, Lin N, et al. The desumoylating enzyme sentrin-specific protease 3 contributes to myocardial ischemia reperfusion injury. J Genet Genomics 2018;45:125–135.
Guo W, Liu X, Li J, Shen Y, Zhou Z, Wang M, et al. Prdx1 alleviates cardiomyocyte apoptosis through ROS-activated MAPK pathway during myocardial ischemia/reperfusion injury. Int J Biol Macromol 2018;112:608–615.
Ravindran S, Boovarahan SR, Shanmugam K, Vedarathinam RC, Kurian GA. Sodium thiosulfate preconditioning ameliorates ischemia/reperfusion injury in rat hearts via reduction of oxidative stress and apoptosis. Cardiovasc Drugs Ther 2017;31:511–524.
Ben-Mahdi MH, Dang PM, Gougerot-Pocidalo MA, O’Dowd Y. Xanthine oxidase-derived ros display a biphasic effect on endothelial cells adhesion and FAK phosphorylation. Oxid Med Cell Longev 2016;2016:9346242.
Seidlmayer LK, Juettner VV, Kettlewell S, Pavlov EV, Blatter LA, Dedkova EN. Distinct mPTP activation mechanisms in ischaemia-reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphosphate Cardiovasc Res 2015;106:237–248.
Andrienko T, Pasdois P, Rossbach A, Halestrap AP. Realtime fluorescence measurements of ROS and (Ca2+) in ischemic / reperfused rat hearts: detectable increases occur only after mitochondrial pore opening and are attenuated by ischemic preconditioning. PLoS One 2016;11:e0167300.
Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014;515:431–435.
Becatti M, Barygina V, Mannucci A, Emmi G. Sirt1 protects against oxidative stress-induced apoptosis in fibroblasts from psoriatic patients: a new insight into the pathogenetic mechanisms of psoriasis. Int J Mol Sci 2018;19:e1572.
Cao MM, Lu X, Liu GD, Su Y, Li YB, Zhou J. Resveratrol attenuates type 2 diabetes mellitus by mediating mitochondrial biogenesis and lipid metabolism via Sirtuin type 1. Exp Ther Med 2018;15:576–584.
Ding M, Lei J, Han H, Li W, Qu Y, Fu E, et al. SIRT1 protects against myocardial ischemia-reperfusion injury via activating eNOS in diabetic rats. Cardiovasc Diabetol 2015;14:143.
Cattelan A, Ceolotto G, Bova S, Albiero M, Kuppusamy M, De Martin S, et al. NAD+-dependent SIRT1 deactivation has a key role on ischemia-reperfusion-induced apoptosis. Vascul Pharmacol 2015;70:35–44.
Bugger H, Witt CN, Bode C. Mitochondrial sirtuins in the heart. Heart Fail Rev 2016;21:519–528.
Boal F, Timotin A, Roumegoux J, Alfarano C, Calise D, Anesia R, et al. Apelin-13 administration protects against ischaemia/reperfusion-mediated apoptosis through the FoxO1 pathway in high-fat diet-induced obesity. Br J Pharmacol 2016;173:1850–1863.
Ning Y, Li Z, Qiu Z. FOXO1 silence aggravates oxidative stress-promoted apoptosis in cardiomyocytes by reducing autophagy. Toxicol Sci 2015;40:637–645.
Ding C, Zou Q, Wang F, Wu H, Wang W, Li H, et al. HGF and BFGF secretion by human adipose-derived stem cells improves ovarian function during natural aging via activation of the SIRT1/FOXO1 signaling pathway. Cell Physiol Biochem 2018;45:1316–1332.
Taka C, Hayashi R, Shimokawa K, Tokui K, Okazawa S, Kambara K, et al. SIRT1 and FOXO1 mRNA expression in PBMC correlates to physical activity in COPD patients. Int J Chron Obstruct Pulmon Dis 2017;12:3237–3244.
Wang B, Ding W, Zhang M, Li H, Guo H, Lin L, et al. Role of FOXO1 in aldosterone-induced autophagy: a compensatory protective mechanism related to podocyte injury. Oncotarget 2016;7:45331–45351.
Ding X, Zhang W, Zhao T, Yan C, Du H. Rab7 GTPase controls lipid metabolic signaling in myeloid-derived suppressor cells. Oncotarget 2017;8:30123–30137.
Acknowledgement
The authors are grateful for facility and field assistance from the Tianjin University of Traditional Chinese Medicine.
Author information
Authors and Affiliations
Contributions
Qi X designed experiments; Sun DW and Gao Q carried out experiments, analyzed experimental results, sequencing data and wrote the manuscript.
Corresponding author
Ethics declarations
The authors confirm that there are no conflicts of interest.
Additional information
Supported by Science and Technology Planning Projects of Science and Technology Commission of Tianjin (No. 18ZXDBSY00080), National Natural Science Foundation of China (No. 81503504), and Key Medical and Health Projects of Health and Family Planning Commission of Tianjin (No. 2015KG110)
Rights and permissions
About this article
Cite this article
Sun, Dw., Gao, Q. & Qi, X. Danshensu Ameliorates Cardiac Ischaemia Reperfusion Injury through Activating Sirt1/FoxO1/Rab7 Signal Pathway. Chin. J. Integr. Med. 26, 283–291 (2020). https://doi.org/10.1007/s11655-019-3165-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-019-3165-9