Abstract
Objective
To investigate the effect of ginsenosides from stems and leaves of ginseng on ethanol-induced lipid deposition in human L02 hepatocytes.
Methods
L02 cells were exposed to ethanol for 36 h and treated with or without ginsenosides. The viability of L02 cells was evaluated by methylthiazolyldiphenyl-tetrazolium bromide assay and the triglyceride (TG) content was detected. Lipid droplets were determined by oil red O staining. Intracellular reactive oxygen species (ROS) production and the mitochondrial membrane potential were tested by flow cytometry. The ATP level was measured by reverse phase high performance liquid chromatography. The expression of cytochrome p450 2E1 (CYP2E1) and peroxisome proliferator-activated receptor α (PPARα) was detected by reverse transcriptase-polymerase chain reaction and Western blotting, respectively.
Results
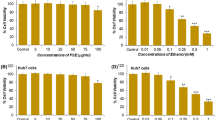
Ethanol exposure resulted in the increase of TG level, lipid accumulation and ROS generation, and the decrease of mitochondrial membrane potential and ATP production in the cells. However, ginsenosides significantly reduced TG content (9.69±0.22 μg/mg protein vs. 4.93±0.49 μg/mg protein, P<0.01), and ROS formation (7254.8±385.7 vs. 5825.2±375.9, P<0.01). Meanwhile, improvements in mitochondrial membrane potential (10655.33±331.34 vs. 11129.52±262.35, P<0.05) and ATP level (1.20±0.18 nmol/mg protein vs. 2.53±0.25 nmol/mg protein, P<0.01) were observed by treatment with ginsenosides. Furthermore, ginsenosides could down-regulate CYP2E1 expression (P<0.01) and upregulate PPARα expression (P<0.01) in ethanol-treated cells.
Conclusions
Ginsenosides could prevent ethanol-induced hepatocyte steatosis in vitro related to the inhibition of oxidative stress and the improvement of mitochondrial function. In addition, the modulation of CYP2E1 and PPARα expression may also play an important role in the protective effect of ginsenosides against lipid accumulation.
Similar content being viewed by others
References
Adachi M, Ishii H. Role of mitochondria in alcoholic liver injury. Free Radic Biol Med 2002;32:487–491.
Dupont I, Lucas D, Clot P, Menez C, Albano E. Cytochrome P4502E1 inducibility and hydroxyethyl radical formation among alcoholics. J Hepatol 1998;28:564–571.
Das SK, Vasudevan DM. Alcohol-induced oxidative stress. Life Sci 2007;81:177–187.
Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med 2008;44:723–738.
Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P4502E1 contributes to ethanol-induced fatty liver in mice. Hepatology 2008;47:1483–1494.
Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 2010;298:G364-G374.
Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferators-activated receptor α (PPAR alpha) agonist treatment reverses PPARa dysfunction and abnormalities in hepatic lipid metabolism in ethanol fed mice. J Biol Chem 2003;278:27997–28004.
Crabb DW, Galli A, Fischer M, You M. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor α. Alcohol 2004;34:35–38.
Xiang YZ, Shang HC, Gao XM, Zhang BL. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother Res 2008;22:851–858.
Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem 2000;203:1–10.
Karu N, Reifen R, Kerem Z. Weight gain reduction in mice fed panax ginseng saponin, a pancreatic lipase inhibitor. J Agric Food Chem 2007;55:2824–2828.
Park EK, Choo MK, Han MJ, Kim DH. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Aller Immunol 2004;133:113–120.
Kang KS, Yamabe N, Kima HY, Okamotob T, Sei Y, Yokoza-wa T. Increase in the free radical scavenging activities of American ginseng by heat processing and its safety evaluation. J Ethnopharmacol 2007;113:225–232.
Hu CF, Lu DX, Sun LP, Qin L. The effect of ginsenosides of stem and leaf on mouse fatty liver and its mechanism. Chin Pharmacol Bull 2009;25:108–112.
Liu W, Zheng Y, Han L, Wang H, Saito M, Ling M, et al. Saponins (Ginsenosides) from stems and leaves of Panax quinquefolium prevented high-fat diet-induced obesity in mice. Phytomedicine 2008;15:1140–1145.
Tilg H, Moschen AR, Kaneider NC. Pathways of liver injury in alcoholic liver disease. J Hepatol 2011;55:1159–1161.
Manzo-Avaloss A, Saavedra-Molina A. Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health 2010;7:4281–4304.
Moon KH, Hood BL, Kim BJ, Hardwick JP, Conrads TP, Veenstra TD, et al. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology 2006;44:1218–1230.
Venkatraman A, Landar A, Davis AJ, Chamlee L, Sanderson T, Kim H, et al. Modification of the mitochondrial proteome in response to the stress of ethanol-dependent hepatotoxicity. J Biol Chem 2004;279:22092–22101.
Kim BJ, Hood BL, Aragon RA, Hardwick JP, Conrads TP, Veenstra TD, et al. Increased oxidation and degradation of cytosolic proteins in alcohol-exposed mouse liver and hepatoma cells. Proteomics 2006;6:1250–1260.
Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med 2008;29:9–16.
Duchen MR, Szabadkai G. Roles of mitochondria in human disease. Essays Biochem 2010;47:115–137.
Murphy MP. How mitochondria produce reactive oxygen species? Biochem J 2009;417:1–13.
Cederbaum AI. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig Dis 2010;28:802–811.
Vasiliou V, Ziegler TL, Bludeau P, Petersen DR, Gonzalez FJ, Deitrich RA. CYP2E1 and catalase influence ethanol sensitivity in the central nervous system. Pharmacogenet Genom 2006;16:51–58.
Wu D, Wang X, Zhou R, Yang L, Cederbaum AI. Alcohol steatosis and cytotoxicity: the role of cytochrome P4502E1 and autophagy. Free Radic Biol Med 2012;53:1346–1357.
Moriya T, Naito H, Ito Y, Nakajima T. Hypothesis of seven balances: molecular mechanisms behind alcoholic liver diseases and association with PPARalpha. J Occup Health 2009;51:391–403.
Hashimoto T, Cook WS, Qi C, Yeldandi AV, Reddy JK, Rao MS. Defect in peroxisome proliferator-activated receptor α-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem 2000;275:28918–28328.
Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, Leclercq I. Central role of PPARα-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology 2003;38:123–132. (Received April 1, 2014; First Online September 10, 2016)
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Natural Science Foundation of Guangdong Province, China (No. S2012010008161)
Rights and permissions
About this article
Cite this article
Hu, Cf., Sun, Lp., Yang, Qh. et al. Ginsenosides from stems and leaves of ginseng prevent ethanol-induced lipid accumulation in human L02 hepatocytes. Chin. J. Integr. Med. 23, 438–444 (2017). https://doi.org/10.1007/s11655-016-2617-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-016-2617-8