Abstract
Objective
To develop and validate a specific patient reported outcome (PRO) for chronic obstructive pulmonary disease (COPD) patients (COPD-PRO) at a set of standardized procedures.
Methods
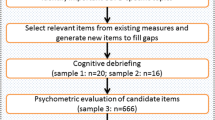
Literature analysis, interview and group discussion were performed to draft an initial model of COPD-PRO. Thereafter, 65 clinicians and experts throughout China reviewed the draft scale. Then cognitive debriefing interviews with 40 patients were conducted to assess respondent comprehension of the scale. After that, the revised scale was validated through pre-testing and field-testing. Finally, the psychometric properties of the COPD-PRO were evaluated by indicators such as validity, reliability and responsiveness based on the data from 230 patients.
Results
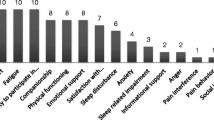
The COPD-PRO contained 17 items in 3 domains: amelioration of clinical symptoms, satisfaction of health condition and satisfaction of treatment effect. The Cronbach's α, Split-half coefficient and test-retest coefficient were 0.806, 0.744, 0.703, respectively; the correlation coefficients between domains and overall scale were 0.835–0.963; 5 factors were extracted according to the conceptual model. The differences of the scale scores before and after treatment were statistically significant (P=0.000).
Conclusions
The COPDPRO has good validity, reliability and responsiveness. The COPD-PRO could provide patients' response to the treatments and then evaluate the effect of treatment in a standardized way.
Similar content being viewed by others
References
Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet 1997;349:1498–1504.
Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev 2009;18:213–221.
National Heart Lung and Blood Institute. Morbidity and mortality: 2009 chart book on cardiovascular, lung, and blood diseases. 1st ed. American: National Heart, Lung, and Blood Institute;2009:62–66.
Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;10:773–778.
Jones PW, Quirk FH, Baveystock CM. The St George's respiratory questionnaire. Respir Med 1991;85:25–31.
Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specificversion of the St. George respiratory questionnaire. Chest 2007;2:456–463.
van der Molen T, Willemse BW, Schokker S, ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the clinical COPD questionnaire. Health Qual Life Outcomes 2003;1:13.
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J 2009;3:648–654.
Revicki DA, Osoba D, Fairclough D, Barofsky I, Berzon R, Leidy NK, et al. Recommendations on health-related quality of life research to support labeling and promotional claims in the United States. Qual Life Res 2000;8:887–900.
Acquadro C, Berzon R, Dubois D, Leidy NK, Marquis P, Revicki D, et al. Incorporating the patient's perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Administration, February 16, 2001. Value Health 2003;5:522–531.
U.S Department of Health and Human Services Food and Drug Administration Guidance for Industry. Patient-reported outcome measures: use in medical product development to support labeling claims. U.S. FDA, clinical/medical (Internet). 2009 Dec (cited 2011 Jan 5). Available from: http://www.fda.gov/downloads/Drugs/GuidanceCompliance RegulatoryInformation/Guidances/UCM193282.pdf
Chen W, Liu JP. Application of patient-reported outcomes in clinical research on therapeutic efficacy. Chin J Integr Tradit West Med (Chin) 2009;8:746–749.
Leung KF, Liu FB, Zhao L, Fang JQ, Chan K, Lin LZ. Development and validation of the Chinese quality of life instrument. Health Qual Life Outcomes 2005;3:26.
Liu FB, Wang WQ. Establishment of the spleen-stomach patients reported outcomes scale in Chinese medicine and the corresponding item selection. World Sci Technol Mod Tradit Chin Med (Chin) 2009;4:527–530.
Li H, Liang WX. Study and development of TCM Stroke Scale for quality of life measurement (1)—establishment of the scale. Liaoning J Tradit Chin Med (Chin) 2008;3:376–378.
Chen XL, Liu FB, Guo L. Development of patient-reported outcome scale for myasthenia gravis: a psychometric test. J Chin Integr Med (Chin) 2010;2:121–125.
The WHOQOL Group. The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med 1995;10:1403–1409.
Jamieson S. Likert scales: how to (ab) use them. Med Educ 2004;12:1217–1218.
Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin Trials 1991;4(Suppl):142S–158S.
Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika 1951;3:297–334.
Terwee CB, Dekker FW, Wiersinga WM, Prummel MF, Bossuyt PM. On assessing responsiveness of healthrelated quality of life instruments: guidelines for instrument evaluation. Qual Life Res 2003;4:349–363.
Donald L, Patrick. Patient-reported outcomes (PROs). An organizing tool for concepts, measures and applications. QoL Newsletter 2003;31:1–6.
Wyrwich KW, Bullinger M, Aaronson N, Hays RD, Patrick DL. Estimating clinically signifi cant differences in quality of life outcomes. Qual Life Res 2005;2:285–295.
Coons SJ, Gwaltney CJ, Hays RD, Lundy JJ, Sloan JA, Revicki DA, et al. Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force report. Value Health 2009;4:419–429.
Fang JQ, ed. The methods and application of quality of life measurement. 1st ed. Beijing: Beijing Medical University Press; 2000:263–266.
Wright JG, Young NL. A comparison of different indices of responsiveness. J Clin Epidemiol 1997;3:239–246.
Author information
Authors and Affiliations
Additional information
Supported by 2011 Special Fund for Chinese Medicine-Scientific Research in the Public Interest of Ministry of Finance People's Republic of China and State Administration of Traditional Chinese Medicine (No. 201107002)
Rights and permissions
About this article
Cite this article
Li, Js., Wang, Mh., Yu, Xq. et al. Development and validation of a patient reported outcome instrument for chronic obstructive pulmonary diseases. Chin. J. Integr. Med. 21, 667–675 (2015). https://doi.org/10.1007/s11655-014-1982-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-014-1982-4