Abstract
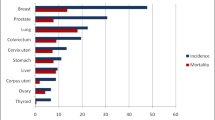
Lung cancer is the leading cause of cancer-related deaths worldwide. Medical imaging technologies such as computed tomography (CT) and positron emission tomography (PET) are routinely used for non-invasive lung cancer diagnosis. In clinical practice, physicians investigate the characteristics of tumors such as the size, shape and location from CT and PET images to make decisions. Recently, scientists have proposed various computational image features that can capture more information than that directly perceivable by human eyes, which promotes the rise of radiomics. Radiomics is a research field on the conversion of medical images into high-dimensional features with data-driven methods to help subsequent data mining for better clinical decision support. Radiomic analysis has four major steps: image preprocessing, tumor segmentation, feature extraction and clinical prediction. Machine learning, including the high-profile deep learning, facilitates the development and application of radiomic methods. Various radiomic methods have been proposed recently, such as the construction of radiomic signatures, tumor habitat analysis, cluster pattern characterization and end-to-end prediction of tumor properties. These methods have been applied in many studies aiming at lung cancer diagnosis, treatment and monitoring, shedding light on future non-invasive evaluations of the nodule malignancy, histological subtypes, genomic properties and treatment responses. In this review, we summarized and categorized the studies on the general workflow, methods for clinical prediction and clinical applications of machine learning in lung cancer radiomic studies, introduced some commonly-used software tools, and discussed the limitations of current methods and possible future directions.
Similar content being viewed by others
References
R. S. Herbst, D. Morgensztern, C. Boshoff. The biology and management of non-small cell lung cancer. Nature, vol. 553, no. 7689, pp. 446–454, 2018. DOI: https://doi.org/10.1038/nature25183.
H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, F. Bray. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, vol. 71, no. 3, pp. 209–249, 2021. DOI: https://doi.org/10.3322/caac.21660.
M. Reck, K. F. Rabe. Precision diagnosis and treatment for advanced non-small-cell lung cancer. The New England Journal of Medicine, vol. 377, no. 9, pp. 849–861, 2017. DOI: https://doi.org/10.1056/NEJMra1703413.
T. W. H. Meijer, L. F. de Geus-Oei, E. P. Visser, W. J. G. Oyen, M. G. Looijen-Salamon, D. Visvikis, A. F. T. M. Verhagen, J. Bussink, D. Vriens. Tumor delineation and quantitative assessment of glucose metabolic rate within histologic subtypes of non-small cell lung cancer by using dynamic.18F fluorodeoxyglucose PET. Radiology, vol. 283, no. 2, pp. 547–559, 2017. DOI: https://doi.org/10.1148/radiol.2016160329.
G. Lee, S. H. Bak, H. Y. Lee. CT radiomics in thoracic oncology: Technique and clinical applications. Nuclear Medicine and Molecular Imaging, vol. 52, no. 2, pp. 91–98, 2018. DOI: https://doi.org/10.1007/s13139-017-0506-5.
P. Lambin, E. Rios-Velazquez, R. Leijenaar, S. Carvalho, R. G. P. M. van Stiphout, P. Granton, C. M. L. Zegers, R. Gillies, R. Boellard, A. Dekker, H. J. W. L. Aerts. Radiomics: Extracting more information from medical images using advanced feature analysis. European Journal of Cancer, vol. 48, no. 4, pp. 441–446, 2012. DOI: https://doi.org/10.1016/j.ejca.2011.11.036.
P. Lambin, R. T. H. Leijenaar, T. M. Deist, J. Peerlings, E. E. C. de Jong, J. van Timmeren, S. Sanduleanu, R. T. H. M. Larue, A. J. G. Even, A. Jochems, Y. van Wijk, H. Woodruff, J. Van Soest, T. Lustberg, E. Roelofs, W. van Elmpt, A. Dekker, F. M. Mottaghy, J. E. Wildberger, S. Walsh. Radiomics: The bridge between medical imaging and personalized medicine. Nature Reviews Clinical Oncology, vol. 14, no. 12, pp. 749–762, 2017. DOI: https://doi.org/10.1038/nrclinonc.2017.141.
R. J. Gillies, P. E. Kinahan, H. Hricak. Radiomics: Images are more than pictures, they are data. Radiology, vol. 278, no. 2, pp. 563–577, 2016. DOI: https://doi.org/10.1148/radiol.2015151169.
Y. LeCun, Y. Bengio, G. Hinton. Deep learning. Nature, vol. 521, no. 7553, pp. 436–444, 2015. DOI: https://doi.org/10.1038/nature14539.
D. S. Kermany, M. Goldbaum, W. J. Cai, C. C. S. Valentim, H. Y. Liang, S. L. Baxter, A. Mckeown, G. Yang, X. K. Wu, F. B. Yan, J. Dong, M. K. Prasadha, J. Pei, M. Y. L. Ting, J. Zhu, C. Li, S. Hewett, J. Dong, I. Ziyar, A. Shi, R. Z. Zhang, L. H. Zheng, R. Hou, W. Shi, X. Fu, Y. O. Duan, V. A. N. Huu, C. Wen, E. D. Zhang, C. L. Zhang, O. L. Li, X. B. Wang, M. A. Singer, X. D. Sun, J. Xu, A. Tafreshi, M. A. Lewis, H. M. Xia, K. Zhang. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell, vol. 172, no. 5, pp. 1122–1131.e9, 1122. DOI: https://doi.org/10.1016/j.cell.2018.02.010.
G. Litjens, T. Kooi, B. E. Bejnordi, A. A. A. Setio, F. Ciompi, M. Ghafoorian, J. A. W. M. Van Der laak, B. Van Ginneken, C. I. Sánchez. A survey on deep learning in medical image analysis. Medical Image Analysis, vol. 42, pp. 60–88, 2017. DOI: https://doi.org/10.1016/j.media.2017.07.005.
M. Diwakar, M. Kumar. A review on CT image noise and its denoising. Biomedical Signal Processing and Control, vol. 42, pp. 73–88, 2018. DOI: https://doi.org/10.1016/j.bspc.2018.01.010.
T. M. Lehmann, C. Gonner, K. Spitzer. Survey: Interpolation methods in medical image processing. IEEE Transactions on Medical Imaging, vol. 18, no. 11, pp. 1049–1075, 1999. DOI: https://doi.org/10.1109/42.816070.
D. Mattes, D. R. Haynor, H. Vesselle, T. K. Lewellen, W. Eubank. PET-CT image registration in the chest using free-form deformations. IEEE Transactions on Medical Imaging, vol. 22, no. 1, pp. 120–128, 2003. DOI: https://doi.org/10.1109/TMI.2003.809072.
H. J. Yu, X. R. Zhou, H. Y. Jiang, H. J. Kang, Z. G. Wang, T. Hara, H. Fujita. Learning 3D non-rigid deformation based on an unsupervised deep learning for PET/CT image registration. In Proceedings of SPIE 10953, Medical Imaging 2019: Biomedical Applications in Molecular, Structural, and Functional Imaging, San Diego, USA, pp.439–444, 2019. DOI: https://doi.org/10.1117/12.2512698.
E. Smistad, T. L. Falch, M. Bozorgi, A. C. Elster, F. Lindseth. Medical image segmentation on GPUs — A comprehensive review. Medical Image Analysis, vol. 20, no. 1, pp. 1–18, 2015. DOI: https://doi.org/10.1016/j.media.2014.10.012.
M. Aljabri, M. AlGhamdi. A review on the use of deep learning for medical images segmentation. Neurocomputing, vol. 506, pp. 311–335, 2022. DOI: https://doi.org/10.1016/j.neucom.2022.07.070.
M. Havaei, A Davy, D. Warde-Farley, A Biard, A Courville, Y. Bengio, C. Pal, P. Jodoin, H. Larochelle. Brain tumor segmentation with Deep Neural Networks. Medical Image Analysis, vol. 35, pp. 18–31, 2017 DOI: https://doi.org/10.1016/j.media.2016.05.004.
T. Messay, R. C. Hardie, S. K. Rogers. A new computationally efficient CAD system for pulmonary nodule detection in CT imagery. Medical Image Analysis, vol. 14, no. 3, pp. 390–406, 2010. DOI: https://doi.org/10.1016/j.media.2010.02.004.
J. J. Suárez-Cuenca, W. Guo, Q. Li. Automated detection of pulmonary nodules in CT: False positive reduction by combining multiple classifiers. In Proceedings of SPIE 7963, Medical Imaging 2011: Computer-Aided Diagnosis, Lake Buena Vista (Orlando), USA, pp. 927–932, 2011. DOI: https://doi.org/10.1117/12.878793.
M. Javaid, M. Javid, M. Z. U. Rehman, S. I. A. Shah. A novel approach to CAD system for the detection of lung nodules in CT images. Computer Methods and Programs in Biomedicine, vol. 135, pp. 125–139, 2016. DOI: https://doi.org/10.1016/j.cmpb.2016.07.031.
J. Long, E. Shelhamer, T. Darrell. Fully convolutional networks for semantic segmentation. In Proceedings of IEEE Conference on Computer Vision and Pattern Recognition, Boston, USA, pp. 3431–3440, 2015. DOI: https://doi.org/10.1109/CVPR.2015.7298965.
K. M. He, G. Gkioxari, P. Dollár, R Girshick. Mask R-CNN. In Proceedings of IEEE International Conference on Computer Vision, Venice, Italy, pp. 2980–2988, 2017. DOI: https://doi.org/10.1109/ICCV.2017.322.
O. Ronneberger, P. Fischer, T. Brox. U-Net: Convolutional networks for biomedical image segmentation. In Proceedings of the 18th International Conference on Medical Image Computing and Computer-assisted Intervention, Springer, Munich, Germany, pp. 234–241, 2015. DOI: https://doi.org/10.1007/978-3-319-24574-4_28.
Ö. Çiçek, A. Abdulkadir, S. S. Lienkamp, T. Brox, O. Ronneberger. 3D U-Net: Learning dense volumetric segmentation from sparse annotation. In Proceedings of the 19th International Conference on Medical Image Computing and Computer-Assisted Intervention, Springer, Athens, Greece, pp. 424–432, 2016. DOI: https://doi.org/10.1007/978-3-319-46723-8_49.
F. Isensee, P. F. Jaeger, S. A. A. Kohl, J. Petersen, K. H. Maier-Hein. nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation. Nature Methods, vol. 18, no. 2, pp. 203–211, 2021. DOI: https://doi.org/10.1038/s41592-020-01008-z.
X. Liu, F. J. Zhang, Z. Y. Hou, L. Mian, Z. Y. Wang, J. Zhang, J. Tang. Self-supervised learning: Generative or contrastive. IEEE Transactions on Knowledge and Data Engineering, to be published. DOI: https://doi.org/10.1109/TKDE.2021.3090866.
L. Chen, P. Bentley, K. Mori, K. Misawa, M. Fujiwara, D. Rueckert. Self-supervised learning for medical image analysis using image context restoration. Medical Image Analysis, vol. 58, Article number 101539, 2019. DOI: https://doi.org/10.1016/j.media.2019.101539.
H. J. Lee, Y. T. Kim, C. H. Kang, B. S. Zhao, Y. Q. Tan, L. H. Schwartz, T. Persigehl, Y. K. Jeon, D. H. Chung. Epidermal growth factor receptor mutation in lung adenocarcinomas: Relationship with CT characteristics and histologic subtypes. Radiology, vol. 268, no. 1, pp. 254–264, 2013. DOI: https://doi.org/10.1148/radiol.13112553.
H. J. W. L. Aerts, E. R. Velazquez, R. T. H. Leijenaar, C. Parmar, P. Grossmann, S. Carvalho, J. Bussink, R. Monshouwer, B. Haibe-Kains, D. Rietveld, F. Hoebers, M. M. Rietbergen, C. R. Leemans, A. Dekker, J. Quackenbush, R. J. Gillies, P. Lambin. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nature Communications, vol. 5, Article number 4006, 2014. DOI: https://doi.org/10.1038/ncomms5006.
M. R. Tomaszewski, R. J. Gillies. The biological meaning of radiomic features. Radiology, vol. 298, no. 3, pp. 505–516, 2021. DOI: https://doi.org/10.1148/radiol.2021202553.
J. J. M. van Griethuysen, A. Fedorov, C. Parmar, A. Hosny, N. Aucoin, V. Narayan, R. G. H. Beets-Tan, J. C. Fillion-Robin, S. Pieper, H. J. W. L. Aerts. Computational radiomics system to decode the radiographic phenotype. Cancer Research, vol. 77, no. 21, pp. e104–e107, 2017. DOI: https://doi.org/10.1158/0008-5472.CAN-17-0339.
A. Dosovitskiy, L. Beyer, A. Kolesnikov, D. Weissenborn, X. H. Zhai, T. Unterthiner, M. Dehghani, M. Minderer, G. Heigold, S. Gelly, J. Uszkoreit, N. Houlsby. An image is worth 16x16 words: Transformers for image recognition at scale, [Online], https://openreview.net/forum?id=YicbFdNTTy, 2022.
A. Vaswani, N. Shazeer, N. Parmar, J. Uszkoreit, L. Jones, A. N. Gomez, L. Kaiser, I. Polosukhin. Attention is all you need. In Proceedings of the 31st Conference on Neural Information Processing Systems, Long Beach, USA, pp. 6000–6010, 2017.
S. Khan, M. Naseer, M. Hayat, S. W. Zamir, F. S. Khan, M. Shah. Transformers in vision: A survey. ACM Computing Surveys, vol. 54, no. 10s, Article number 200, 2022. DOI: https://doi.org/10.1145/3505244.
F. Shamshad, S. Khan, S. W. Zamir, M. H. Khan, M. Hayat, F. S. Khan, H. Z. Fu. Transformers in medical imaging: A survey. [Online], Available: http://arxiv.org/abs/2201.09873, 2022.
N. Papanikolaou, C. Matos, D. M. Koh. How to develop a meaningful radiomic signature for clinical use in oncologic patients. Cancer Imaging, vol. 20, no. 1, Article number 33, 2020. DOI: https://doi.org/10.1186/s40644-020-00311-4.
R. Tibshirani. Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society: Series B (Methodological), vol. 58, no. 1, pp. 267–288, 1996. DOI: https://doi.org/10.1111/j.2517-6161.1996.tb02080.x.
H. J. W. L. Aerts. The potential of radiomic-based phenotyping in precision medicine: A review. JAMA Oncology, vol. 2, no. 12, Article number 1636, 2016. DOI: https://doi.org/10.1001/jamaoncol.2016.2631.
X. G. Zhang, X. Lu, Q. Shi, X. Q. Xu, H. C. E. Leung, L. N. Harris, J. D. Iglehart, A. Miron, J. S. Liu, W. H. Wong. Recursive SVM feature selection and sample classification for mass-spectrometry and microarray data. BMC Bioinformatics, vol. 7, Article number 197, 2006. DOI: https://doi.org/10.1186/1471-2105-7-197.
I. Guyon, J. Weston, S. Barnhill, V. Vapnik. Gene selection for cancer classification using support vector machines. Machine Learning, vol. 46, no. 1, pp. 389–422, 2002. DOI: https://doi.org/10.1023/A:1012487302797.
D. R. Cox. Regression models and life-tables. Journal of the Royal Statistical Society: Series B (Methodological), vol. 34, no. 2, pp. 187–202, 1972. DOI: https://doi.org/10.1111/j.2517-6161.1972.tb00899.x.
K. Bera, N. Braman, A. Gupta, V. Velcheti, A. Madabhushi. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nature Reviews Clinical Oncology, vol. 19, no. 2, pp. 132–146, 2022. DOI: https://doi.org/10.1038/s41571-021-00560-7.
H. Kimura, R. D. Braun, E. T. Ong, R. Hsu, T. W. Secomb, D. Papahadjopoulos, K. Hong, M. W. Dewhirst. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Research, vol. 56, no. 23, pp. 5522–5528, 1996.
P. Carmeliet, R. K. Jain. Molecular mechanisms and clinical applications of angiogenesis. Nature, vol. 473, no. 7347, pp. 298–307, 2011. DOI: https://doi.org/10.1038/nature10144.
M. R. Junttila, F. J. de Sauvage. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature, vol. 501, no. 7467, pp. 346–354, 2013. DOI: https://doi.org/10.1038/nature12626.
S. Napel, W. Mu, B. V. Jardim-Perassi, H. J. W. L. Aerts, R. J. Gillies. Quantitative imaging of cancer in the post-genomic era: Radio(geno)mics, deep learning, and habitats. Cancer, vol. 124, no. 24, pp. 4633–4649, 2018. DOI: https://doi.org/10.1002/cncr.31630.
J. Wu, M. F. Gensheimer, X. Z. Dong, D. L. Rubin, S. Napel, M. Diehn, B. W. Loo, R. J. Li. Robust intratumor partitioning to identify high-risk subregions in lung cancer: A pilot study. International Journal of Radiation Oncology · Biology · Physics, vol. 95, no. 5, pp. 1504–1512, 2016. DOI: https://doi.org/10.1016/j.ijrobp.2016.03.018.
A. J. G. Even, B. Reymen, M. D. La Fontaine, M. Das, F. M. Mottaghy, J. S. A. Belderbos, D. De Ruysscher, P. Lambin, W. van Elmpt. Clustering of multi-parametric functional imaging to identify high-risk subvolumes in non-small cell lung cancer. Radiotherapy and Oncology, vol. 125, no. 3, pp. 379–384, 2017. DOI: https://doi.org/10.1016/j.radonc.2017.09.041.
R. Achanta, A. Shaji, K. Smith, A. Lucchi, P. Fua, S. Süsstrunk. SLIC superpixels compared to state-of-the-art superpixel methods. IEEE Transactions on Pattern Analysis and Machine Intelligence, vol. 34, no. 11, pp. 2274–2282, 2012. DOI: https://doi.org/10.1109/TPAMI.2012.120.
L. Chen, K. F. Liu, X. Zhao, H. Shen, K. Zhao, W. T. Zhu. Habitat imaging-based.18F-FDG PET/CT radiomics for the preoperative discrimination of non-small cell lung cancer and benign inflammatory diseases. Frontiers in Oncology, vol. 11, Article number 759897, 2021. DOI: https://doi.org/10.3389/fonc.2021.759897.
H. Shen, L. Chen, K. F. Liu, K. Zhao, J. S. Li, L. J. Yu, H. W. Ye, W. T. Zhu. A subregion-based positron emission tomography/computed tomography (PET/CT) radiomics model for the classification of non-small cell lung cancer histopathological subtypes. Quantitative Imaging in Medicine and Surgery, vol. 11, no. 7, pp. 2918–2932, 2021. DOI: https://doi.org/10.21037/qims-20-1182.
D. Cherezov, D. Goldgof, L. Hall, R. Gillies, M. Schabath, H. Müller, A. Depeursinge. Revealing tumor habitats from texture heterogeneity analysis for classification of lung cancer malignancy and aggressiveness. Scientific Reports, vol. 9, no. 1, Article number 4500, 2019. DOI: https://doi.org/10.1038/s41598-019-38831-0.
A. Marusyk, M. Janiszewska, K. Polyak. Intratumor heterogeneity: The Rosetta stone of therapy resistance. Cancer Cell, vol. 37, no. 4, pp. 471–484, 2020. DOI: https://doi.org/10.1016/j.ccell.2020.03.007.
J. Q. Li, H. M. Lu, X. Fang, S. J. Chen, X. G. Zhang. Pixel-level clustering reveals intra-tumor heterogeneity in non-small cell lung cancer. In Proceedings of IEEE International Conference on Bioinformatics and Biomedicine, San Diego, USA, pp.1536–1539, 2019. DOI: https://doi.org/10.1109/BIBM47256.2019.8983174.
T. Caliński, J. Harabasz. A dendrite method for cluster analysis. Communications in Statistics, vol. 3, no. 1, pp. 1–27, 1974. DOI: https://doi.org/10.1080/03610927408827101.
R. Tibshirani, G. Walther, T. Hastie. Estimating the number of clusters in a data set via the gap statistic. Journal of the Royal Statistical Society: Series B (Statistical Methodology), vol. 63, no. 2, pp. 411–423, 2001. DOI: https://doi.org/10.1111/1467-9868.00293.
A. Hosny, C. Parmar, J. Quackenbush, L. H. Schwartz, H. J. W. L. Aerts. Artificial intelligence in radiology. Nature Reviews Cancer, vol. 18, no. 8, pp. 500–510, 2018. DOI: https://doi.org/10.1038/s41568-018-0016-5.
D. E. Rumelhart, G. E. Hinton, R. J. Williams. Learning representations by back-propagating errors. Nature, vol. 323, no. 6088, pp. 533–536, 1986. DOI: https://doi.org/10.1038/323533a0.
P. Mobadersany, S. Yousefi, M. Amgad, D. A. Gutman, J. S. Barnholtz-Sloan, J. E. Velázquez Vega, D. J. Brat, L. A. D. Cooper. Predicting cancer outcomes from histology and genomics using convolutional networks. Proceedings of the National Academy of Sciences of the United States of America, vol.115, no. 13, pp. E2970–E2979, 2018. DOI: https://doi.org/10.1073/pnas.1717139115.
G. Chartrand, P. M. Cheng, E. Vorontsov, M. Drozdzal, S. Turcotte, C. J. Pal, S. Kadoury, A. Tang. Deep learning: A primer for radiologists. RadioGraphics, vol. 37, no. 7, pp. 2113–2131, 2017. DOI: https://doi.org/10.1148/rg.2017170077.
J. Deng, W. Dong, R. Socher, L. J. Li, K. Li, L. Fei-Fei. ImageNet: A large-scale hierarchical image database. In Proceedings of IEEE Conference on Computer Vision and Pattern Recognition, Miami, USA, pp. 248–255, 2009. DOI: https://doi.org/10.1109/CVPR.2009.5206848.
W. Shen, M. Zhou, F. Yang, D. Dong, C. Y. Yang, Y. L. Zang, J. Tian. Learning from experts: Developing transferable deep features for patient-level lung cancer prediction. In Proceedings of the 19th International Conference on Medical Image Computing and Computer-assisted Intervention, Springer, Athens, Greece, pp. 124–131, 2016. DOI: https://doi.org/10.1007/978-3-319-46723-8_15.
Y. W. Xu, A. Hosny, R. Zeleznik, C. Parmar, T. Coroller, I. Franco, R. H. Mak, H. J. W. L. Aerts. Deep learning predicts lung cancer treatment response from serial medical imaging. Clinical Cancer Research, vol. 25, no. 11, pp. 3266–3275, 2019. DOI: https://doi.org/10.1158/1078-0432.CCR-18-2495.
C. Larson. China’s AI imperative. Science, vol. 359, no. 6376, pp. 628–630, 2018. DOI: https://doi.org/10.1126/science.359.6376.628.
The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England Journal of Medicine, vol. 365, no. 5, pp. 395–409, 2011. DOI: https://doi.org/10.1056/NEJMoa1102873.
S. Hawkins, H. Wang, Y. Liu, A. Garcia, O. Stringfield, H. Krewer, Q. Li, D. Cherezov, R. A. Gatenby, Y. Balagurunathan, D. Goldgof, M. B. Schabath, L. Hall, R. J. Gillies. Predicting malignant nodules from screening CT scans. Journal of Thoracic Oncology, vol. 11, no. 12, pp. 2120–2128, 2016. DOI: https://doi.org/10.1016/j.jtho.2016.07.002.
W. Wu, L. A. Pierce, Y. Z. Zhang, S. N. J. Pipavath, T. W. Randolph, K. J. Lastwika, P. D. Lampe, A. M. Houghton, H. N. Liu, L. M. Xia, P. E. Kinahan. Comparison of prediction models with radiological semantic features and radiomics in lung cancer diagnosis of the pulmonary nodules: A case-control study. European Radiology, vol. 29, no. 11, pp. 6100–6108, 2019. DOI: https://doi.org/10.1007/s00330-019-06213-9.
Y. T. Xie, Y. Xia, J. P. Zhang, Y. Song, D. G. Feng, M. Fulham, W. D. Cai. Knowledge-based collaborative deep learning for benign-malignant lung nodule classification on chest CT. IEEE Transactions on Medical Imaging, vol. 38, no. 4, pp. 991–1004, 2019. DOI: https://doi.org/10.1109/TMI.2018.2876510.
J. Wang, X. Liu, D. Dong, J. D. Song, M. Xu, Y. L. Zang, J. Tian. Prediction of malignant and benign of lung tumor using a quantitative radiomic method. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Orlando, USA, pp. 1272–1275, 2016. DOI: https://doi.org/10.1109/EMBC.2016.7590938.
N. Beig, M. Khorrami, M. Alilou, P. Prasanna, N. Braman, M. Orooji, S. Rakshit, K. Bera, P. Rajiah, J. Ginsberg, C. Donatelli, R. Thawani, M. Yang, F. Jacono, P. Tiwari, V. Velcheti, R. Gilkeson, P. Linden, A. Madabhushi. Perinodular and intranodular radiomic features on lung CT images distinguish adenocarcinomas from granulomas. Radiology, vol. 290, no. 3, pp. 783–792, 2019. DOI: https://doi.org/10.1148/radiol.2018180910.
W. Shen, M. Zhou, F. Yang, D. D. Yu, D. Dong, C. Y. Yang, Y. L. Zang, J. Tian. Multi-crop convolutional neural networks for lung nodule malignancy suspiciousness classification. Pattern Recognition, vol. 61, pp. 663–673, 2017. DOI: https://doi.org/10.1016/j.patcog.2016.05.029.
R. P. Zhang, L. Zhu, Z. T. Cai, W. Jiang, J. Li, C. W. Yang, C. X. Yu, B. Jiang, W. Wang, W. G. Xu, X. F. Chai, X. D. Zhang, Y. Tang. Potential feature exploration and model development based on.18F-FDG PET/CT images for differentiating benign and malignant lung lesions. European Journal of Radiology, vol. 121, Article number 108735, 2019. DOI: https://doi.org/10.1016/j.ejrad.2019.108735.
S. Chen, S. Harmon, T. Perk, X. N. Li, M. J. Chen, Y. M. Li, R. Jeraj. Diagnostic classification of solitary pulmonary nodules using dual time.18F-FDG PET/CT image texture features in granuloma-endemic regions.. Scientific Reports, vol. 7, no. 1, Article number 9370, 2017. DOI: https://doi.org/10.1038/s41598-017-08764-7.
F. Kang, W. Mu, J. Gong, S. J. Wang, G. Q. Li, G. Y. Li, W. Qin, J. Tian, J. Wang. Integrating manual diagnosis into radiomics for reducing the false positive rate of.18F-FDG PET/CT diagnosis in patients with suspected lung cancer. European Journal of Nuclear Medicine and Molecular Imaging, vol. 46, no. 13, pp. 2770–2779, 2019. DOI: https://doi.org/10.1007/s00259-019-04418-0.
Y. J. Park, D. Choi, J. Y. Choi, S. H. Hyun. Performance evaluation of a deep learning system for differential diagnosis of lung cancer with conventional CT and FDG PET/CT using transfer learning and metadata. Clinical Nuclear Medicine, vol. 46, no. 8, pp. 635–640, 2021. DOI: https://doi.org/10.1097/RLU.0000000000003661.
R. S. Herbst, J. V. Heymach, S. M. Lippman. Lung cancer. The New England Journal of Medicine, vol. 359, no. 13, pp. 1367–1380, 2008. DOI: https://doi.org/10.1056/NEJMra0802714.
Y. Qu, K. Emoto, T. Eguchi, R. G. Aly, H. Zheng, J. E. Chaft, K. S. Tan, D. R. Jones, M. G. Kris, P. S. Adusumilli, W. D. Travis. Pathologic assessment after neoadjuvant chemotherapy for NSCLC: Importance and implications of distinguishing adenocarcinoma from squamous cell carcinoma. Journal of Thoracic Oncology, vol. 14, no. 3, pp. 482–493, 2019. DOI: https://doi.org/10.1016/j.jtho.2018.11.017.
T. S. Mok, Y. L. Wu, S. Thongprasert, C. H. Yang, D. T. Chu, N. Saijo, P. Sunpaweravong, B. H. Han, B. Margono, Y. Ichinose, Y. Nishiwaki, Y. Ohe, J. J. Yang, B. Chewaskulyong, H. Y. Jiang, E. L. Duffield, C. L. Watkins, A. A. Armour, M. Fukuoka. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England Journal of Medicine, vol. 361, no. 10, pp. 947–957, 2009. DOI: https://doi.org/10.1056/NEJMoa0810699.
W. M. Wu, C. Parmar, P. Grossmann, J. Quackenbush, P. Lambin, J. Bussink, R. Mak, H. J. W. L. Aerts. Exploratory study to identify radiomics classifiers for lung cancer histology. Frontiers in Oncology, vol. 6, Article number 71, 2016. DOI: https://doi.org/10.3389/fonc.2016.00071.
X. Z. Zhu, D. Dong, Z. D. Chen, M. J. Fang, L. W. Zhang, J. D. Song, D. D. Yu, Y. L. Zang, Z. Y. Liu, J. Y. Shi, J. Tian. Radiomic signature as a diagnostic factor for histologic subtype classification of non-small cell lung cancer. European Radiology, vol. 28, no. 7, pp. 2772–2778, 2018. DOI: https://doi.org/10.1007/s00330-017-5221-1.
U. Bashir, B. Kawa, M. Siddique, S. M. Mak, A. Nair, E. Mclean, A. Bille, V. Goh, G. Cook. Non-invasive classification of non-small cell lung cancer: A comparison between random forest models utilising radiomic and semantic features. The British Journal of Radiology, vol. 92, no. 1099, Article number 20190159, 2019. DOI: https://doi.org/10.1259/bjr.20190159.
X. Sha, G. Z. Gong, Q. T. Qiu, J. H. Duan, D. W. Li, Y. Yin. Identifying pathological subtypes of non-small-cell lung cancer by using the radiomic features of.18F-fluorodeoxyglucose positron emission computed tomography. Translational Cancer Research, vol. 8, no. 5, pp. 1741–1749, 2019. DOI: https://doi.org/10.21037/tcr.2019.08.20.
S. H. Hyun, M. S. Ahn, Y. W. Koh, S. J. Lee. A machine-learning approach using PET-based radiomics to predict the histological subtypes of lung cancer. Clinical Nuclear Medicine, vol. 44, no. 12, pp. 956–960, 2019. DOI: https://doi.org/10.1097/RLU.0000000000002810.
S. Koyasu, M. Nishio, H. Isoda, Y. Nakamoto, K. Togashi. Usefulness of gradient tree boosting for predicting histological subtype and EGFR mutation status of non-small cell lung cancer on.18F FDG-PET/CT. Annals of Nuclear Medicine, vol. 34, no. 1, pp. 49–57, 2020. DOI: https://doi.org/10.1007/s12149-019-01414-0.
T. L. Chaunzwa, A. Hosny, Y. W. Xu, A. Shafer, N. Diao, M. Lanuti, D. C. Christiani, R. H. Mak, H. J. W. L. Aerts. Deep learning classification of lung cancer histology using CT images. Scientific Reports, vol. 11, no. 1, Article number 5471, 2021. DOI: https://doi.org/10.1038/s41598-021-84630-x.
P. Marentakis, P. Karaiskos, V. Kouloulias, N. Kelekis, S. Argentos, N. Oikonomopoulos, C. Loukas. Lung cancer histology classification from CT images based on radiomics and deep learning models. Medical & Biological Engineering & Computing, vol. 59, no. 1, pp. 215–226, 2021. DOI: https://doi.org/10.1007/s11517-020-02302-w.
Y. X. Guo, Q. Song, M. M. Jiang, Y. L. Guo, P. Xu, Y. Q. Zhang, C. C. Fu, Q. Fang, M. S. Zeng, X. Z. Yao. Histological subtypes classification of lung cancers on CT images using 3D deep learning and radiomics. Academic Radiology, vol. 28, no. 9, pp. e258–e266, 2021. DOI: https://doi.org/10.1016/j.acra.2020.06.010.
L. Ubaldi, V. Valenti, R. F. Borgese, G. Collura, M. E. Fantacci, G. Ferrera, G. Iacoviello, B. F. Abbate, F. Laruina, A. Tripoli, A. Retico, M. Marrale. Strategies to develop radiomics and machine learning models for lung cancer stage and histology prediction using small data samples. Physica Medica, vol. 90, pp. 13–22, 2021. DOI: https://doi.org/10.1016/j.ejmp.2021.08.015.
J. Liu, J. J. Cui, F. Liu, Y. X. Yuan, F. Guo, G. L. Zhang. Multi-subtype classification model for non-small cell lung cancer based on radiomics: SLS model. Medical Physics, vol. 46, no. 7, pp. 3091–3100, 2019. DOI: https://doi.org/10.1002/mp.13551.
R. Patil, G. Mahadevaiah, A. Dekker. An approach toward automatic classification of tumor histopathology of non-small cell lung cancer based on radiomic features. Tomography, vol. 2, no. 4, pp. 374–377, 2016. DOI: https://doi.org/10.18383/j.tom.2016.00244.
W. D. Travis, E. Brambilla, A. G. Nicholson, Y. Yatabe, J. H. M. Austin, M. B. Beasley, L. R. Chirieac, S. Dacic, E. Duhig, D. B. Flieder, K. Geisinger, F. R. Hirsch, Y. Ishikawa, K. M. Kerr, M. Noguchi, G. Pelosi, C. A. Powell, M. S. Tsao, I. Wistuba. The 2015 world health organization classification of lung tumors. Journal of Thoracic Oncology, vol. 10, no. 9, pp. 1243–1260, 2015. DOI: https://doi.org/10.1097/JTO.0000000000000630.
C. Zhang, J. J. Zhang, F. P. Xu, Y. G. Wang, Z. Xie, J. Su, S. Dong, Q. Nie, Y. Shao, Q. Zhou, J. J. Yang, X. N. Yang, X. C. Zhang, Z. Li, Y. L. Wu, W. Z. Zhong. Genomic landscape and immune microenvironment features of preinvasive and early invasive lung adenocarcinoma. Journal of Thoracic Oncology, vol. 14, no. 11, pp. 1912–1923, 2019. DOI: https://doi.org/10.1016/j.jtho.2019.07.031.
J. Gong, J. Y. Liu, W. Hao, S. D. Nie, S. P. Wang, W. J. Peng. Computer-aided diagnosis of ground-glass opacity pulmonary nodules using radiomic features analysis. Physics in Medicine & Biology, vol. 64, no. 13, Article number 135015, 2019. DOI: https://doi.org/10.1088/1361-6560/ab2757.
X. Wang, Q. C. Li, J. L. Cai, W. Wang, P. Xu, Y. Q. Zhang, Q. Fang, C. C. Fu, L. Fan, Y. Xiao, S. Y. Liu. Predicting the invasiveness of lung adenocarcinomas appearing as ground-glass nodule on CT scan using multi-task learning and deep radiomics. Translational Lung Cancer Research, vol. 9, no. 4, pp. 1397–1406, 2020. DOI: https://doi.org/10.21037/tlcr-20-370.
Y. L. She, L. Zhang, H. Y. Zhu, C. Y. Dai, D. Xie, H. K. Xie, W. Zhang, L. L. Zhao, L. L. Zou, K. Fei, X. W. Sun, C. Chen. The predictive value of CT-based radiomics in differentiating indolent from invasive lung adenocarcinoma in patients with pulmonary nodules. European Radiology, vol. 28, no. 12, pp. 5121–5128, 2018. DOI: https://doi.org/10.1007/s00330-018-5509-9
M. Yuan, Y. D. Zhang, X. H. Pu, Y. Zhong, H. Li, J. F. Wu, T. F. Yu. Comparison of a radiomic biomarker with volumetric analysis for decoding tumour phenotypes of lung adenocarcinoma with different disease-specific survival. European Radiology, vol. 27, no. 11, pp. 4857–4865, 2017. DOI: https://doi.org/10.1007/s00330-017-4855-3.
H. H. Cho, G. Lee, H. Y. Lee, H. Park. Marginal radiomics features as imaging biomarkers for pathological invasion in lung adenocarcinoma. European Radiology, vol. 30, no. 5, pp. 2984–2994, 2020. DOI: https://doi.org/10.1007/s00330-019-06581-2.
F. Y. Xu, W. C. Zhu, Y. Shen, J. Wang, R. Xu, C. Outesh, L. J. Song, Y. Gan, C. L. Pu, H. J. Hu. Radiomic-based quantitative CT analysis of pure ground-glass nodules to predict the invasiveness of lung adenocarcinoma. Frontiers in Oncology, vol. 10, Article number 872, 2020. DOI: https://doi.org/10.3389/fonc.2020.00872.
L. Y. Wu, C. Gao, J. F. Ye, J. Y. Tao, N. Wang, P. P. Pang, P. Xiang, M. S. Xu. The value of various peritumoral radiomic features in differentiating the invasiveness of adenocarcinoma manifesting as ground-glass nodules. European Radiology, vol. 31, no. 12, pp. 9030–9037, 2021. DOI: https://doi.org/10.1007/s00330-021-07948-0.
J. Cai, H. Liu, H. Yuan, Y. Wu, Q. Xu, Y. Lv, J. Li, J. Fu, J. Ye. A radiomics study to predict invasive pulmonary adenocarcinoma appearing as pure ground-glass nodules. Clinical Radiology, vol. 76, no. 2, pp. 143–151, 2021. DOI: https://doi.org/10.1016/j.crad.2020.10.005.
W. F. Chen, M. Li, D. B. Mao, X. J. Ge, J. F. Wang, M. Y. Tan, W. L. Ma, X. M. Huang, J. J. Lu, C. Li, Y. Q. Hua, H. Wu. Radiomics signature on CECT as a predictive factor for invasiveness of lung adenocarcinoma manifesting as subcentimeter ground glass nodules. Scientific Reports, vol. 11, no. 1, Article number 3633, 2021. DOI: https://doi.org/10.1038/s41598-021-83167-3.
V. L. Cox, P. Bhosale, G. R. Varadhachary, N. Wagner-Bartak, I. C. Glitza, K. A. Gold, J. T. Atkins, P. T. Soliman, D. S. Hong, A. Qayyum. Cancer genomics and important oncologic mutations: A contemporary guide for body imagers. Radiology, vol. 283, no. 2, pp. 314–340, 2017. DOI: https://doi.org/10.1148/radiol.2017152224.
E. A. Ashley. Towards precision medicine. Nature Reviews Genetics, vol. 17, no. 9, pp. 507–522, 2016. DOI: https://doi.org/10.1038/nrg.2016.86.
W. Mu, L. Jiang, J. Y. Zhang, Y. Shi, J. E. Gray, I. Tunali, C. Gao, Y. Y. Sun, J. Tian, X. M. Zhao, X. L. Sun, R. J. Gillies, M. B. Schabath. Non-invasive decision support for NSCLC treatment using PET/CT radiomics. Nature Communications, vol. 11, no. 1, Article number 5228, 2020. DOI: https://doi.org/10.1038/s41467-020-19116-x.
A. Marusyk, V. Almendro, K. Polyak. Intra-tumour heterogeneity: A looking glass for cancer? Nature Reviews Cancer, vol. 12, no. 5, pp.323–334, 2012. DOI: https://doi.org/10.1038/nrc3261.
N. McGranahan, C. Swanton. Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell, vol. 168, no. 4, pp. 613–628, 2017. DOI: https://doi.org/10.1016/j.cell.2017.01.018.
L. M. Sholl, D. L. Aisner, M. Varella-Garcia, L. D. Berry, D. Dias-Santagata, I. I. Wistuba, H. D. Chen, J. Fujimoto, K. Kugler, W. A. Franklin, A. J. Iafrate, M. Ladanyi, M. G. Kris, B. E. Johnson, P. A. Bunn, J. D. Minna, D. J. Kwiatkowski. Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: The lung cancer mutation consortium experience. Journal of Thoracic Oncology, vol. 10, no. 5, pp. 768–777, 2015. DOI: https://doi.org/10.1097/JTO.0000000000000516.
Y. L. Wu, D. Planchard, S. Lu, H. Sun, N. Yamamoto, D. W. Kim, D. S. W. Tan, J. C. H. Yang, M. Azrif, T. Mitsudomi, K. Park, R. A. Soo, J. W. C. Chang, A. Alip, S. Peters, J. Y. Douillard. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Annals of Oncology, vol. 30, no. 2, pp. 171–210, 2019. DOI: https://doi.org/10.1093/annonc/mdy554.
S. Rizzo, F. Petrella, V. Buscarino, F. De Maria, S. Raimondi, M. Barberis, C. Fumagalli, G. Spitaleri, C. Rampinelli, F. De Marinis, L. Spaggiari, M. Bellomi. CT radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung cancer. European Radiology, vol. 26, no. 1, pp. 32–42, 2016. DOI: https://doi.org/10.1007/s00330-015-3814-0.
Y. N. Qi, T. T. Zhao, M. Y. Han. The application of radiomics in predicting gene mutations in cancer. European Radiology, vol. 32, no. 6, pp. 4014–4024, 2022. DOI: https://doi.org/10.1007/s00330-021-08520-6.
L. Song, Z. C. Zhu, L. Mao, X. L. Li, W. Han, H. Y. Du, H. W. Wu, W. Song, Z. Y. Jin. Clinical, conventional CT and radiomic feature-based machine learning models for predicting ALK rearrangement status in lung adenocarcinoma patients. Frontiers in Oncology, vol. 10, Article number 369, 2020. DOI: https://doi.org/10.3389/fonc.2020.00369.
W. T. Tu, G. Y. Sun, L. Fan, Y. Wang, Y. Xia, Y. Guan, Q. Li, D. Zhang, S. Y. Liu, Z. B. Li. Radiomics signature: A potential and incremental predictor for EGFR mutation status in NSCLC patients, comparison with CT morphology. Lung Cancer, vol. 132, pp. 28–35, 2019. DOI: https://doi.org/10.1016/j.lungcan.2019.03.025.
C. Chang, X. Y. Sun, G. Wang, H. Yu, W. L. Zhao, Y. Q. Ge, S. F. Duan, X. H. Qian, R. Wang, B. Lei, L. H. Wang, L. Liu, M. M. Ruan, H. Yan, C. Y. Liu, J. Chen, W. H. Xie. A machine learning model based on PET/CT radiomics and clinical characteristics predicts ALK rearrangement status in lung adenocarcinoma. Frontiers in Oncology, vol. 11, Article number 603882, 2021. DOI: https://doi.org/10.3389/fonc.2021.603882.
J. Y. Zhang, X. M. Zhao, Y. Zhao, J. M. Zhang, Z. Q. Zhang, J. F. Wang, Y. C. Wang, M. Dai, J. Y. Han. Value of pre-therapy.18F-FDG PET/CT radiomics in predicting EGFR mutation status in patients with non-small cell lung cancer. European Journal of Nuclear Medicine and Molecular Imaging, vol. 47, no. 5, pp. 1137–1146, 2020. DOI: https://doi.org/10.1007/s00259-019-04592-1.
S. Wang, J. Y. Shi, Z. X. Ye, D. Dong, D. D. Yu, M. Zhou, Y. Liu, O. Gevaert, K. Wang, Y. B. Zhu, H. Y. Zhou, Z. Y. Liu, J. Tian. Predicting EGFR mutation status in lung adenocarcinoma on computed tomography image using deep learning. European Respiratory Journal, vol. 53, no. 3, Article number 1800986, 2019. DOI: https://doi.org/10.1183/13993003.00986-2018.
Y. Y. Dong, L. N. Hou, W. K. Yang, J. H. Han, J. W. Wang, Y. Qiang, J. J. Zhao, J. X. Hou, K. Song, Y. L. Ma, N. G. F. Kazihise, Y. F. Cui, X. T. Yang. Multichannel multi-task deep learning for predicting EGFR and KRAS mutations of non-small cell lung cancer on CT images. Quantitative Imaging in Medicine and Surgery, vol. 11, no. 6, pp. 2354–2375, 2021. DOI: https://doi.org/10.21037/qims-20-600.
C. D. Wang, X. Y. Xu, J. Shao, K. Zhou, K. F. Zhao, Y. Q. He, J. W. Li, J. X. Guo, Z. Yi, W. M. Li. Deep learning to predict EGFR mutation and PD-L1 expression status in non-small-cell lung cancer on computed tomography images. Journal of Oncology, vol. 2021, Article number 5499385, 2021. DOI: https://doi.org/10.1155/2021/5499385.
C. D. Wang, J. C. Ma, J. Shao, S. Zhang, Z. N. Liu, Y. Z. Yu, W. M. Li. Predicting EGFR and PD-L1 status in NSCLC patients using multitask AI system based on CT images. Frontiers in Immunology, vol. 13, Article number 813072, 2022. DOI: https://doi.org/10.3389/fimmu.2022.813072.
W. Zhao, J. C. Yang, B. B. Ni, D. X. Bi, Y. L. Sun, M. D. Xu, X. X. Zhu, C. Li, L. Jin, P. Gao, P. J. Wang, Y. Q. Hua, M. Li. Toward automatic prediction of EGFR mutation status in pulmonary adenocarcinoma with 3D deep learning. Cancer Medicine, vol. 8, no. 7, pp. 3532–3543, 2019. DOI: https://doi.org/10.1002/cam4.2233.
L. S. Wang, S. Wang, H. Yu, Y. B. Zhu, W. M. Li, J. Tian. A quarter-split domain-adaptive network for EGFR gene mutation prediction in lung cancer by standardizing heterogeneous CT image. In Proceedings of the 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society, Mexico, pp. 3646–3649, 2021. DOI: https://doi.org/10.1109/EMBC46164.2021.9630395.
B. H. Zhang, S. L. Qi, X. H. Pan, C. Li, Y. D. Yao, W. Qian, Y. B. Guan. Deep CNN model using CT radiomics feature mapping recognizes EGFR gene mutation status of lung adenocarcinoma. Frontiers in Oncology, vol. 10, Article number 598721, 2021. DOI: https://doi.org/10.3389/fonc.2020.598721.
D. Q. Gui, Q. L. Song, B. Song, H. C. Li, M. H. Wang, X. H. Min, A. Li. AIR-Net: A novel multi-task learning method with auxiliary image reconstruction for predicting EGFR mutation status on CT images of NSCLC patients. Computers in Biology and Medicine, vol. 141, Article number 105157, 2022. DOI: https://doi.org/10.1016/j.compbiomed.2021.105157.
G. T. Yin, Z. Y. Wang, Y. C. Song, X. F. Li, Y. W. Chen, L. Zhu, Q. Su, D. Dai, W. G. Xu. Prediction of EGFR mutation status based on.18F-FDG PET/CT imaging using deep learning-based model in lung adenocarcinoma. Frontiers in Oncology, vol. 11, Article number 709137, 2021. DOI: https://doi.org/10.3389/fonc.2021.709137.
A. Subramanian, P. Tamayo, V. K. Mootha, S. Mukherjee, B. L. Ebert, M. A. Gillette, A. Paulovich, S. L. Pomeroy, T. R. Golub, E. S. Lander, J. P. Mesirov. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America, vol. 102, no. 43, pp. 15545–15550, 2005. DOI: https://doi.org/10.1073/pnas.0506580102.
M. Kanehisa, M. Furumichi, M. Tanabe, Y. Sato, K. Morishima. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Research, vol. 45, no. D1, pp. D353–D361, 2017. DOI: https://doi.org/10.1093/nar/gkw1092.
A. Liberzon, C. Birger, H. Thorvaldsdóttir, M. Ghandi, J. P. Mesirov, P. Tamayo. The molecular signatures database hallmark gene set collection. Cell Systems, vol. 1, no. 6, pp. 417–425, 2015. DOI: https://doi.org/10.1016/j.cels.2015.12.004.
P. Grossmann, O. Stringfield, N. El-Hachem, M. M. Bui, E. Rios Velazquez, C. Parmar, R. T. Leijenaar, B. Haibe-Kains, P. Lambin, R. J. Gillies, H. J. Aerts. Defining the biological basis of radiomic phenotypes in lung cancer. eLife, vol. 6, Article number e23421, 2017. DOI: https://doi.org/10.7554/eLife.23421.
J. Lee, Y. Cui, X. L. Sun, B. L. Li, J. Wu, D. W. Li, M. F. Gensheimer, B. W. Loo, M. Diehn, R. J. Li. Prognostic value and molecular correlates of a CT image-based quantitative pleural contact index in early stage NSCLC. European Radiology, vol. 28, no. 2, pp. 736–746, 2018. DOI: https://doi.org/10.1007/s00330-017-4996-4.
T. Xia, A. Kumar, M. Fulham, D. G. Feng, Y. Wang, E. Y. Kim, Y. Jung, J. Kim. Fused feature signatures to probe tumour radiogenomics relationships. Scientific Reports, vol. 12, no. 1, Article number 2173, 2022. DOI: https://doi.org/10.1038/s41598-022-06085-y.
N. F. Smedley, D. R. Aberle, W. Hsu. Using deep neural networks and interpretability methods to identify gene expression patterns that predict radiomic features and histology in non-small cell lung cancer. Journal of Medical Imaging, vol. 8, no. 3, Article number 031906, 2021. DOI: https://doi.org/10.1117/1.JMI.8.3.031906.
O. Gevaert, J. J. Xu, C. D. Hoang, A. N. Leung, Y. Xu, A. Quon, D. L. Rubin, S. Napel, S. K. Plevritis. Non-small cell lung cancer: Identifying prognostic imaging biomarkers by leveraging public gene expression microarray data-methods and preliminary results. Radiology, vol. 264, no. 2, pp. 387–396, 2012. DOI: https://doi.org/10.1148/radiol.12111607.
M. Zhou, A. Leung, S. Echegaray, A. Gentles, J. B. Shrager, K. C. Jensen, G. J. Berry, S. K. Plevritis, D. L. Rubin, S. Napel, O. Gevaert. Non-small cell lung cancer radiogenomics map identifies relationships between molecular and imaging phenotypes with prognostic implications. Radiology, vol. 286, no. 1, pp. 307–315, 2018. DOI: https://doi.org/10.1148/radiol.2017161845
T. Wang, J. Gong, H. H. Duan, L. J. Wang, X. D. Ye, S. D. Nie. Correlation between CT based radiomics features and gene expression data in non-small cell lung cancer. Journal of X-Ray Science and Technology, vol. 27, no. 5, pp. 773–803, 2019. DOI: https://doi.org/10.3233/XST-190526.
J. Li, Y. X. Liu, W. L. Dong, Y. Zhou, J. Q. Wu, K. Luan, L. S. Qi. Identifying.18F-FDG PET-metabolic radiomic signature for lung adenocarcinoma prognosis via the leveraging of prognostic transcriptomic module. Quantitative Imaging in Medicine and Surgery, vol. 12, no. 3, pp. 1893–1908, 2022. DOI: https://doi.org/10.21037/qims-21-706.
P. Langfelder, S. Horvath. WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics, vol. 9, Article number 559, 2008. DOI: https://doi.org/10.1186/1471-2105-9-559.
L. Shui, H. Y. Ren, X. Yang, J. Li, Z. W. Chen, C. Yi, H. Zhu, P. X. Shui. The era of radiogenomics in precision medicine: An emerging approach to support diagnosis, treatment decisions, and prognostication in oncology. Frontiers in Oncology, vol. 10, Article number 570465, 2021. DOI: https://doi.org/10.3389/fonc.2020.570465.
N. Shah, J. Q. Li, F. H. Li, W. C. Chen, H. X. Gao, S. J. Chen, K. Hua, X. G. Zhang. An experiment on ab initio discovery of biological knowledge from scRNA-Seq data using machine learning. Patterns, vol. 1, no. 5, Article number 100071, 2020. DOI: https://doi.org/10.1016/j.patter.2020.100071.
D. A. Lawson, K. Kessenbrock, R. T. Davis, N. Pervolarakis, Z. Werb. Tumour heterogeneity and metastasis at single-cell resolution. Nature Cell Biology, vol. 20, no. 12, pp. 1349–1360, 2018. DOI: https://doi.org/10.1038/s41556-018-0236-7.
F. Y. Wu, J. Fan, Y. Y. He, A. W. Xiong, J. Yu, Y. X. Li, Y. Zhang, W. C. Zhao, F. Zhou, W. Li, J. Zhang, X. S. Zhang, M. Qiao, G. H. Gao, S. H. Chen, X. X. Chen, X. F. Li, L. K. Hou, C. Y. Wu, C. X. Su, S. X. Ren, M. Odenthal, R. Buettner, N. Fang, C. C. Zhou. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nature Communications, vol. 12, no. 1, Article number 2540, 2021. DOI: https://doi.org/10.1038/s41467-021-22801-0.
Y. Q. Huang, Z. Y. Liu, L. He, X. Chen, D. Pan, Z. L. Ma, C. S. Liang, J. Tian, C. H. Liang. Radiomics signature: A potential biomarker for the prediction of disease-free survival in early-stage (I or II) non-small cell lung cancer. Radiology, vol. 281, no. 3, pp. 947–957, 2066. DOI: https://doi.org/10.1148/radiol.2016152234.
A. Hosny, C. Parmar, T. P. Coroller, P. Grossmann, R. Zeleznik, A. Kumar, J. Bussink, R. J. Gillies, R. H. Mak, H. J. W. L. Aerts. Deep learning for lung cancer prognostication: A retrospective multi-cohort radiomics study. PLoS Medicine, vol. 15, no. 11, Article number e1002711, 2018. DOI: https://doi.org/10.1371/journal.pmed.1002711.
T. P. Coroller, V. Agrawal, E. Huynh, V. Narayan, S. W. Lee, R. H. Mak, H. J. W. L. Aerts. Radiomic-based pathological response prediction from primary tumors and lymph nodes in NSCLC. Journal of Thoracic Oncology, vol. 12, no. 3, pp. 467–476, 2017. DOI: https://doi.org/10.1016/j.jtho.2016.11.2226.
N. S. Zhang, R. Liang, M. F. Gensheimer, M. Y. Guo, H. Zhu, J. M. Yu, M. Diehn, B. W. Loo, R. J. Li, J. Wu. Early response evaluation using primary tumor and nodal imaging features to predict progression-free survival of locally advanced non-small cell lung cancer. Theranostics, vol. 10, no. 25, pp. 11707–11718, 2020. DOI: https://doi.org/10.7150/thno.50565.
P. Vaidya, K. Bera, A. Gupta, X. X. Wang, G. Corredor, P. F. Fu, N. Beig, P. Prasanna, P. D. Patil, P. D. Velu, P. Rajiah, R. Gilkeson, M. D. Feldman, H. Choi, V. Velcheti, A. Madabhushi. CT derived radiomic score for predicting the added benefit of adjuvant chemotherapy following surgery in stage I, II resectable non-small cell lung cancer: A retrospective multicohort study for outcome prediction. The Lancet Digital Health, vol. 2, no. 3, pp. e116–e128, 2020. DOI: https://doi.org/10.1016/S2589-7500(20)30002-9.
M. Khorrami, P. Jain, K. Bera, M. Alilou, R. Thawani, P. Patil, U. Ahmad, S. Murthy, K. Stephans, P. F. Fu, V. Velcheti, A. Madabhushi. Predicting pathologic response to neoadjuvant chemoradiation in resectable stage III non-small cell lung cancer patients using computed tomography radiomic features. Lung Cancer, vol. 135, pp. 1–9, 2019. DOI: https://doi.org/10.1016/j.lungcan.2019.06.020.
P. Jain, M. Khorrami, A. Gupta, P. Rajiah, K. Bera, V. S. Viswanathan, P. F. Fu, A. Dowlati, A. Madabhushi. Novel non-invasive radiomic signature on CT scans predicts response to platinum-based chemotherapy and is prognostic of overall survival in small cell lung cancer. Frontiers in Oncology, vol. 11, Article number 744724, 2021. DOI: https://doi.org/10.3389/fonc.2021.744724.
F. C. Yang, J. Y. Zhang, L. Zhou, W. Xia, R. Zhang, H. F. Wei, J. X. Feng, X. Y. Zhao, J. M. Jian, X. Gao, S. H. Yuan. CT-based radiomics signatures can predict the tumor response of non-small cell lung cancer patients treated with first-line chemotherapy and targeted therapy. European Radiology, vol. 32, no. 3, pp. 1538–1547, 2022. DOI: https://doi.org/10.1007/s00330-021-08277-y.
R. S. Chang, S. L. Qi, Y. Yue, X. Y. Zhang, J. D. Song, W. Qian. Predictive radiomic models for the chemotherapy response in non-small-cell lung cancer based on computerized-tomography images. Frontiers in Oncology, vol. 11, Article number 646190, 2021. DOI: https://doi.org/10.3389/fonc.2021.646190.
E. E. C. de Jong, W. van Elmpt, S. Rizzo, A. Colarieti, G. Spitaleri, R. T. H. Leijenaar, A. Jochems, L. E. L. Hendriks, E. G. C. Troost, B. Reymen, A. M. C. Dingemans, P. Lambin. Applicability of a prognostic CT-based radiomic signature model trained on stage I-III non-small cell lung cancer in stage IV non-small cell lung cancer. Lung Cancer, vol. 124, pp. 6–11, 2018. DOI: https://doi.org/10.1016/j.lungcan.2018.07.023.
D. Xie, T. T. Wang, S. J. Huang, J. J. Deng, Y. J. Ren, Y. Yang, J. Q. Wu, L. Zhang, K. Fei, X. W. Sun, Y. L. She, C. Chen. Radiomics nomogram for prediction disease-free survival and adjuvant chemotherapy benefits in patients with resected stage I lung adenocarcinoma. Translational Lung Cancer Research, vol. 9, no. 4, pp. 1112–1123, 2020. DOI: https://doi.org/10.21037/tlcr-19-577.
M. Khorrami, M. Khunger, A. Zagouras, P. Patil, R. Thawani, K. Bera, P. Rajiah, P. F. Fu, V. Velcheti, A. Madabhushi. Combination of peri- and intratumoral radiomic features on baseline CT scans predicts response to chemotherapy in lung adenocarcinoma. Radiology: Artificial Intelligence, vol. 1, no. 2, Article number 180012, 2019. DOI: https://doi.org/10.1148/ryai.2019180012.
D. V. Fried, O. Mawlawi, L. F. Zhang, X. Fave, S. H. Zhou, G. Ibbott, Z. X. Liao, L. E. Court. Stage III non-small cell lung cancer: Prognostic value of FDG PET quantitative imaging features combined with clinical prognostic factors. Radiology, vol. 278, no. 1, pp. 214–222, 2016. DOI: https://doi.org/10.1148/radiol.2015142920.
J. Wu, T. Aguilera, D. Shultz, M. Gudur, D. L. Rubin, B. W. J. Loo, M. Diehn, R. J. Li. Early-stage non-small cell lung cancer: Quantitative imaging characteristics of.18F fluorodeoxyglucose PET/CT allow prediction of distant metastasis. Radiology, vol. 281, no. 1, pp. 270–278, 2016. DOI: https://doi.org/10.1148/radiol.2016151829.
Z. C. Jiao, H. M. Li, Y. Xiao, J. Dorsey, C. B. Simone, S. Feigenberg, G. Kao, Y. Fan. Integration of deep learning radiomics and counts of circulating tumor cells improves prediction of outcomes of early stage NSCLC patients treated with stereotactic body radiation therapy. International Journal of Radiation Oncology Biology Physics, vol. 112, no. 4, pp. 1045–1054, 2022. DOI: https://doi.org/10.1016/j.ijrobp.2021.11.006.
J. E. van Timmeren, W. van Elmpt, R. T. H. Leijenaar, B. Reymen, R. Monshouwer, J. Bussink, L. Paelinck, E. Bogaert, C. De Wagter, E. Elhaseen, Y. Lievens, O. Hansen, C. Brink, P. Lambin. Longitudinal radiomics of cone-beam CT images from non-small cell lung cancer patients: Evaluation of the added prognostic value for overall survival and locoregional recurrence. Radiotherapy and Oncology, vol. 136, pp. 78–85, 2019. DOI: https://doi.org/10.1016/j.radonc.2019.03.032.
E. Huynh, T. P. Coroller, V. Narayan, V. Agrawal, Y. Hou, J. Romano, I. Franco, R. H. Mak, H. J. W. L. Aerts. CT-based radiomic analysis of stereotactic body radiation therapy patients with lung cancer. Radiotherapy and Oncology, vol. 120, no. 2, pp. 258–266, 2016. DOI: https://doi.org/10.1016/j.radonc.2016.05.024.
H. M. Li, M. Galperin-Aizenberg, D. Pryma, C. B. Simone, Y. Fan. Unsupervised machine learning of radiomic features for predicting treatment response and overall survival of early stage non-small cell lung cancer patients treated with stereotactic body radiation therapy. Radiotherapy and Oncology, vol. 129, no. 2, pp. 218–226, 2018. DOI: https://doi.org/10.1016/j.radonc.2018.06.025.
L. L. Wang, T. T. Dong, B. W. Xin, C. R. Xu, M. Y. Guo, H. Q. Zhang, D. G. Feng, X. Y. Wang, J. M. Yu. Integrative nomogram of CT imaging, clinical, and hematological features for survival prediction of patients with locally advanced non-small cell lung cancer. European Radiology, vol. 29, no. 6, pp. 2958–2967, 2019. DOI: https://doi.org/10.1007/s00330-018-5949-2.
M. A. Arshad, A. Thornton, H. N. Lu, H. Tam, K. Wallitt, N. Rodgers, A. Scarsbrook, G. Mcdermott, G. J. Cook, D. Landau, S. Chua, R. O’connor, J. Dickson, D. A Power, T. D. Barwick, A Rockall, E. O. Aboagye. Discovery of pre-therapy 2-deoxy-2-18F-fluoro-D-glucose positron emission tomography-based radiomics classifiers of survival outcome in non-small-cell lung cancer patients European Journal of Nuclear Medicine and Molecular Imaging, vol. 46, no. 2, pp. 455–466, 2019. DOI: https://doi.org/10.1007/s00259-018-4139-4.
L. M. Luo, B. T. Huang, C. Z. Chen, Y. Wang, C. H. Su, G. B. Peng, C. B. Zeng, Y. X. Wu, R. H. Wang, K. Huang, Z. H. Qiu. A combined model to improve the prediction of local control for lung cancer patients undergoing stereotactic body radiotherapy based on radiomic signature plus clinical and dosimetric parameters. Frontiers in Oncology, vol. 11, Article number 819047, 2022. DOI: https://doi.org/10.3389/fonc.2021.819047.
Q. Li, J. Kim, Y. Balagurunathan, J. Qi, Y. Liu, K. Latifi, E. G. Moros, M. B. Schabath, Z. X. Ye, R. J. Gillies, T. J. Dilling. CT imaging features associated with recurrence in non-small cell lung cancer patients after stereotactic body radiotherapy. Radiation Oncology, vol. 12, no. 1, Article number 158, 2017. DOI: https://doi.org/10.1186/s13014-017-0892-y.
B. Liang, H. Yan, Y. Tian, X. Y. Chen, L. L. Yan, T. Zhang, Z. M. Zhou, L. Wang, J. R. Dai. Dosiomics: Extracting 3D spatial features from dose distribution to predict incidence of radiation pneumonitis. Frontiers in Oncology, vol. 9, Article number 269, 2019. DOI: https://doi.org/10.3389/fonc.2019.00269.
B. Lou, S. Doken, T. L. Zhuang, D. Wingerter, M. Gidwani, N. Mistry, L. Ladic, A. Kamen, M. E. Abazeed. An image-based deep learning framework for individualising radiotherapy dose: A retrospective analysis of outcome prediction. The Lancet Digital Health, vol. 1, no. 3, pp. e136–e147, 2019. DOI: https://doi.org/10.1016/S2589-7500(19)30058-5.
A. M. Barragán-Montero, D. Nguyen, W. G. Lu, M. H. Lin, R. Norouzi-Kandalan, X. Geets, E. Sterpin, S. Jiang. Three-dimensional dose prediction for lung IMRT patients with deep neural networks: Robust learning from heterogeneous beam configurations. Medical Physics, vol. 46, no. 8, pp. 3679–3691, 2019. DOI: https://doi.org/10.1002/mp.13597.
Y. Li, K. H. He, M. W. Ma, X. Qi, Y. Bai, S. W. Liu, Y. Gao, F. Lyu, C. H. Jia, B. Zhao, X. S. Gao. Using deep learning to model the biological dose prediction on bulky lung cancer patients of partial stereotactic ablation radiotherapy. Medical Physics, vol. 47, no. 12, pp. 6540–6550, 2020. DOI: https://doi.org/10.1002/mp.14518.
M. Maemondo, A. Inoue, K. Kobayashi, S. Sugawara, S. Oizumi, H. Isobe, A. Gemma, M. Harada, H. Yoshizawa, I. Kinoshita, Y. Fujita, S. Okinaga, H. Hirano, K. Yoshimori, T. Harada, T. Ogura, M. Ando, H. Miyazawa, T. Tanaka, Y. Saijo, K. Hagiwara, S. Morita, T. Nukiwa, North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. The New England Journal of Medicine, vol. 362, no. 25, pp. 2380–2388, 2010. DOI: https://doi.org/10.1056/NEJMoa0909530.
J. D. Song, L. Wang, N. N. Ng, M. F. Zhao, J. Y. Shi, N. Wu, W. M. Li, Z. Y. Liu, K. W. Yeom, J. Tian. Development and validation of a machine learning model to explore tyrosine kinase inhibitor response in patients with stage IV EGFR variant-positive non-small cell lung cancer. JAMA Network Open, vol. 3, no. 12, Article number e2030442, 2020. DOI: https://doi.org/10.1001/jamanetworkopen.2020.30442.
S. Wang, H. Yu, Y. C. Gan, Z. J. Wu, E. C. Li, X. H. Li, J. X. Cao, Y. B. Zhu, L. S. Wang, H. Deng, M. Xie, Y. Y. Wang, X. D. Ma, D. Liu, B. J. Chen, P. W. Tian, Z. X. Qiu, J. H. Xian, J. Ren, K. Wang, W. Wei, F. Xie, Z. H. Li, Q. Wang, X. Y. Xue, Z. Y. Liu, J. Y. Shi, W. M. Li, J. Tian. Mining whole-lung information by artificial intelligence for predicting EGFR genotype and targeted therapy response in lung cancer: A multicohort study. The Lancet Digital Health, vol. 4, no. 5, pp. e309–e319, 2022. DOI: https://doi.org/10.1016/S2589-7500(22)00024-3.
L. Huang, J. Y. Chen, W. G. Hu, X. Y. Xu, D. Liu, J. M. Wen, J. Y. Lu, J. Z. Cao, J. H. Zhang, Y. Gu, J. Z. Wang, M. Fan. Assessment of a radiomic signature developed in a general NSCLC cohort for predicting overall survival of ALK-positive patients with different treatment types. Clinical Lung Cancer, vol. 20, no. 6, pp. e638–e651, 2019. DOI: https://doi.org/10.1016/j.cllc.2019.05.005.
Z. B. Song, T. C. Liu, L. Shi, Z. Y. Yu, Q. Shen, M. D. Xu, Z. Z. Huang, Z. J. Cai, W. X. Wang, C. W. Xu, J. J. Sun, M. Chen. The deep learning model combining CT image and clinicopathological information for predicting ALK fusion status and response to ALK-TKI therapy in non-small cell lung cancer patients. European Journal of Nuclear Medicine and Molecular Imaging, vol. 48, no. 2, pp. 361–371, 2021. DOI: https://doi.org/10.1007/s00259-020-04986-6.
H. L. Li, R. Zhang, S. W. Wang, M. J. Fang, Y. B. Zhu, Z. H. Hu, D. Dong, J. Y. Shi, J. Tian. CT-based radiomic signature as a prognostic factor in stage IV ALK-positive non-small-cell lung cancer treated with TKI crizotinib: A proof-of-concept study. Frontiers in Oncology, vol. 10, Article number 57, 2020. DOI: https://doi.org/10.3389/fonc.2020.00057.
X. Tang, Y. Li, W. F. Yan, W. L. Qian, T. Pang, Y. L. Gong, Z. G. Yang. Machine learning-based CT radiomics analysis for prognostic prediction in metastatic non-small cell lung cancer patients with EGFR-T790M mutation receiving third-generation EGFR-TKI osimertinib treatment. Frontiers in Oncology, vol. 11, Article number 719919, 2021. DOI: https://doi.org/10.3389/fonc.2021.719919.
R. P. Hou, X. Y. Li, J. F. Xiong, T. L. Shen, W. Yu, L. H. Schwartz, B. S. Zhao, J. Zhao, X. L. Fu. Predicting tyrosine kinase inhibitor treatment response in stage IV lung adenocarcinoma patients with EGFR mutation using model-based deep transfer learning. Frontiers in Oncology, vol. 11, Article number 679764, 2021. DOI: https://doi.org/10.3389/fonc.2021.679764.
D. Shao, D. Y. Du, H. P. Liu, J. Q. Lv, Y. Cheng, H. Zhang, W. B. Lv, S. X. Wang, L. J. Lu. Identification of stage IIIC/IV EGFR-mutated non-small cell lung cancer populations sensitive to targeted therapy based on a PET/CT radiomics risk model. Frontiers in Oncology, vol. 11, Article number 721318, 2021. DOI: https://doi.org/10.3389/fonc.2021.721318.
S. Kruger, M. Ilmer, S. Kobold, B. L. Cadilha, S. Endres, S. Ormanns, G. Schuebbe, B. W. Renz, J. G. D’haese, H. Schloesser, V. Heinemann, M. Subklewe, S. Boeck, J. Werner, M. von Bergwelt-baildon. Advances in cancer immunotherapy 2019 — latest trends. Journal of Experimental & Clinical Cancer Research, vol. 38, no. 1, Article number 268, 2019. DOI: https://doi.org/10.1186/s13046-019-1266-0.
D. W. S. Mak, S. Li, A. Minchom. Challenging the recalcitrant disease-developing molecularly driven treatments for small cell lung cancer. European Journal of Cancer, vol. 119, pp. 132–150, 2019. DOI: https://doi.org/10.1016/j.ejca.2019.04.037.
F. F. Teng, X. J. Meng, L. Kong, J. M. Yu. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: A systematic review. Cancer Letters, vol. 414, pp. 166–173, 2018. DOI: https://doi.org/10.1016/j.canlet.2017.11.014.
C. Zhang, L. de A. F. Fonseca, Z. W. Shi, C. Zhu, A. Dekker, I. Bermejo, L. Wee. Systematic review of radiomic biomarkers for predicting immune checkpoint inhibitor treatment outcomes. Methods, vol. 188, pp. 61–72, 2021. DOI: https://doi.org/10.1016/j.ymeth.2020.11.005.
L. Fehrenbacher, A. Spira, M. Ballinger, M. Kowanetz, J. Vansteenkiste, J. Mazieres, K. Park, D. Smith, A. Artal-Cortes, C. Lewanski, F. Braiteh, D. Waterkamp, P. He, W. Zou, D. S. Chen, J. Yi, A. Sandler, A. Rittmeyer. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. The Lancet, vol. 387, no. 10030, pp. 1837–1846, 2016. DOI: https://doi.org/10.1016/S0140-6736(16)00587-0.
K. Jazieh, M. Khorrami, A. Saad, M. Gad, A. Gupta, P. Patil, V. S. Viswanathan, P. Rajiah, C. J. Nock, M. Gilkey, P. F. Fu, N. A. Pennell, A. Madabhushi. Novel imaging biomarkers predict outcomes in stage III unresectable non-small cell lung cancer treated with chemoradiation and durvalumab. Journal for ImmunoTherapy of Cancer, vol. 10, no. 3, Article number e003778, 2022. DOI: https://doi.org/10.1136/jitc-2021-003778.
Y. Liu, M. H. Wu, Y. W. Zhang, Y. H. Luo, S. He, Y. N. Wang, F. Chen, Y. L. Liu, Q. Yang, Y. Y. Li, H. Wei, H. Zhang, C. W. Jin, N. Lu, W. H. Li, S. C. Wang, Y. Guo, Z. X. Ye. Imaging biomarkers to predict and evaluate the effectiveness of immunotherapy in advanced non-small-cell lung cancer. Frontiers in Oncology, vol. 11, Article number 657615, 2021. DOI: https://doi.org/10.3389/fonc.2021.657615.
B. X. He, D. Dong, Y. L. She, C. C. Zhou, M. J. Fang, Y. B. Zhu, H. H. Zhang, Z. P. Huang, T. Jiang, J. Tian, C. Chen. Predicting response to immunotherapy in advanced non-small-cell lung cancer using tumor mutational burden radiomic biomarker. Journal for ImmunoTherapy of Cancer, vol. 8, no. 2, Article number e000550, 2020. DOI: https://doi.org/10.1136/jitc-2020-000550.
W. Mu, L. Jiang, Y. Shi, I. Tunali, J. E. Gray, E. Katsoulakis, J. Tian, R. J. Gillies, M. B. Schabath. Non-invasive measurement of PD-L1 status and prediction of immunotherapy response using deep learning of PET/CT images. Journal for ImmunoTherapy of Cancer, vol. 9, no. 6, Article number e002118, 2021. DOI: https://doi.org/10.1136/jitc-2020-002118.
C. Park, K. J. Na, H. Choi, C. Y. Ock, S. Ha, M. Kim, S. Park, B. Keam, T. M. Kim, J. C. Paeng, I. K. Park, C. H. Kang, D. W. Kim, G. J. Cheon, K. W. Kang, Y. T. Kim, D. S. Heo. Tumor immune profiles noninvasively estimated by FDG PET with deep learning correlate with immunotherapy response in lung adenocarcinoma. Theranostics, vol. 10, no. 23, pp. 10838–10848, 2020. DOI: https://doi.org/10.7150/thno.50283.
B. Yang, L. Zhou, J. Zhong, T. F. Lv, A. Li, L. Ma, J. Zhong, S. S. Yin, L. T. Huang, C. S. Zhou, X. Y. Li, Y. Q. Ge, X. W. Tao, L. J. Zhang, Y. Son, G. M. Lu. Combination of computed tomography imaging-based radiomics and clinicopathological characteristics for predicting the clinical benefits of immune checkpoint inhibitors in lung cancer. Respiratory Research, vol. 22, no. 1, Article number 189, 2021. DOI: https://doi.org/10.1186/s12931-021-01780-2.
S. Trebeschi, S. G. Drago, N. J. Birkbak, I. Kurilova, A. M. Călin, A. Delli Pizzi, F. Lalezari, D. M. J. Lambregts, M. W. Rohaan, C. Parmar, E. A. Rozeman, K. J. Hartemink, C. Swanton, J. B. A. G. Haanen, C. U. Blank, E. F. Smit, R. G. H. Beets-Tan, H. J. W. L. Aerts. Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Annals of Oncology, vol. 30, no. 6, pp. 998–1004, 2019. DOI: https://doi.org/10.1093/annonc/mdz108.
M. H. Wu, Y. Y. Zhang, J. N. Zhang, Y. W. Zhang, Y. N. Wang, F. Chen, Y. H. Luo, S. He, Y. L. Liu, Q. Yang, Y. Y. Li, H. Wei, H. Zhang, N. Lu, S. C. Wang, Y. Guo, Z. X. Ye, Y. Liu. A combined-radiomics approach of CT images to predict response to anti-PD-1 immunotherapy in NSCLC: A retrospective multicenter study. Frontiers in Oncology, vol. 11, Article number 688679, 2022. DOI: https://doi.org/10.3389/fonc.2021.688679.
I. Tunali, J. E. Gray, J. Qi, M. Abdalah, D. K. Jeong, A. Guvenis, R. J. Gillies, M. B. Schabath. Novel clinical and radiomic predictors of rapid disease progression phenotypes among lung cancer patients treated with immunotherapy: An early report. Lung Cancer, vol. 129, pp. 75–79, 2019. DOI: https://doi.org/10.1016/j.lungcan.2019.01.010.
M. Khorrami, P. Prasanna, A. Gupta, P. Patil, P. D. Velu, R. Thawani, G. Corredor, M. Alilou, K. Bera, P. F. Fu, M. Feldman, V. Velcheti, A. Madabhushi. Changes in CT radiomic features associated with lymphocyte distribution predict overall survival and response to immunotherapy in non-small cell lung cancer. Cancer Immunology Research, vol. 8, no. 1, pp. 108–119, 2020. DOI: https://doi.org/10.1158/2326-6066.CIR-19-0476.
P. A. Yushkevich, J. Piven, H. C. Hazlett, R. G. Smith, S. Ho, J. C. Gee, G. Gerig. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage, vol. 31, no. 3, pp. 1116–1128, 2006. DOI: https://doi.org/10.1016/j.neuroimage.2006.01.015.
A. Fedorov, R. Beichel, J. Kalpathy-Cramer, J. Finet, J. C. Fillion-Robin, S. Pujol, C. Bauer, D. Jennings, F. Fennessy, M. Sonka, J. Buatti, S. Aylward, J. V. Miller, S. Pieper, R. Kikinis. 3D Slicer as an image computing platform for the quantitative imaging network. Magnetic Resonance Imaging, vol. 30, no. 9, pp. 1323–1341, 2012. DOI: https://doi.org/10.1016/j.mri.2012.05.001.
D. Mason, scaramallion, mrbean-bremen, rhaxton, J. Suever, Vanessasaurus, D. P. Orfanos, G. Lemaitre, A. Panchal, A. Rothberg, M. D. Herrmann, J. Massich, J. Kerns, K. van Golen, T. Robitaille, S. Biggs, moloney, C. Bridge, M. Shun-Shin, B. Conrad, pawelzajdel, M. Mattes, Y. Lyu, F. C. Morency, T. Cogan, H. Meine, J. Wortmann. pydicom/pydicom: Pydicom 2.3.0: Zenodo, [Online], Available: https://doi.org/10.5281/zenodo.6394735, July 28, 2022.
M. Brett, C. J. Markiewicz, M. Hanke, M. A. Côté, B. Cipollini, P. McCarthy, D. Jarecka, C. P. Cheng, Y. O. Halchenko, M. Cottaar, E. Larson, S. Ghosh, D. Wassermann, S. Gerhard, G. R. Lee, H. T. Wang, E. Kastman, J. Kaczmarzyk, R. Guidotti, J. Daniel, O. Duek, A. Rokem, C. Madison, B. Moloney, F. C. Morency, M. Goncalves, R. Markello, C. Riddell, A. Sólon, C. Burns, J. Millman, A. Gramfort, J. Leppäkangas, J. J. F. van den Bosch, R. D. Vincent, H. Braun, K. Subramaniam, D. Papadopoulos Orfanos, A. Van, K. J. Gorgolewski, P. R. Raamana, J. Klug, B. N. Nichols, E. M. Baker, S. Hayashi, B. Pinsard, C. Haselgrove, M. Hymers, O. Esteban, S. Koudoro, F. Pérez-Garc’ia, J. Dockès, N. N. Oosterhof, B. Amirbekian, I. Nimmo-Smith, L. Nguyen, S. Reddigari, S. St-Jean, E. Panfilov, F. Garyfallidis, G. Varoquaux, J. H. Legarreta, K. S. Hahn, L. Waller, O. P. Hinds, B. Fauber, J. Roberts, J. B. Poline, J. Stutters, K. Jordan, M. Cieslak, M. E. Moreno, T. Hrnčiar, V. Haenel, Y. Schwartz, Z. Baratz, B. C. Darwin, B. Thirion, C. Gauthier, I. Solovey, I. Gonzalez, J. Palasubramaniam, J. Lecher, K. Leinweber, K. Raktivan, M. Calábková, P. Fischer, P. Gervais, S. Gadde, T. Ballinger, T. Roos, V. R. Reddam. nipy/nibabel: Zenodo, [Online], Available: https://doi.org/10.5281/zenodo.6658382, 2022.
B. C. Lowekamp, D. T. Chen, L. Ibáñez, D. Blezek. The design of SimpleITK. Frontiers in Neuroinformatics, vol. 7, Article number 45, 2013. DOI: https://doi.org/10.3389/fninf.2013.00045.
G. Bradski. The OpenCV library. Dr. Dobb’s Journal: Software Tools for the Professional Programmer, vol. 25, no. 11, pp. 120–123, 2000.
P. Virtanen, R. Gommers, T. E. Oliphant, M. Haberland, T. Reddy, D. Cournapeau, E. Burovski, P. Peterson, W. Weckesser, J. Bright, S. J. van der walt, M. Brett, J. Wilson, K. J. Millman, N. Mayorov, A. R. J. Nelson, E. Jones, R. Kern, E. Larson, C. J. Carey, İ. Polat, Y. Feng, E. W. Moore, J. Vanderplas, D. Laxalde, J. Perktold, R. Cimrman, I. Henriksen, E. A. Quintero, C. R. Harris, A. M. Archibald, A. H. Ribeiro, F. Pedregosa, P. van Mulbregt. SciPy 1.0 Contributors. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nature Methods, vol. 17, no. 3, pp. 261–272, 2020. DOI: https://doi.org/10.1038/s41592-019-0686-2.
K. Marstal, F. Berendsen, M. Staring, S. Klein. SimpleElastix: A user-friendly, multi-lingual library for medical image registration. In Proceedings of IEEE Conference on Computer Vision and Pattern Recognition Workshops, Las Vegas, USA, pp. 574–582, 2016. DOI: https://doi.org/10.1109/CVPRW.2016.78.
M. P. Heinrich, M. Jenkinson, M. Brady, J. A. Schnabel. MRF-based deformable registration and ventilation estimation of lung CT. IEEE Transactions on Medical Imaging, vol. 32, no. 7, pp. 1239–1248, 2013. DOI: https://doi.org/10.1109/TMI.2013.2246577.
F. Pedregosa, G. Varoquaux, A. Gramfort, V. Michel, B. Thirion, O. Grisel, M. Blondel, P. Prettenhofer, R. Weiss, V. Dubourg, J. Vanderplas, A. Passos, D. Cournapeau, M. Brucher, M. Perrot, É. Duchesnay. Scikit-learn: Machine learning in python. The Journal of Machine Learning Research, vol. 12, pp. 2825–2830, 2011.
A. Paszke, S. Gross, F. Massa, A. Lerer, J. Bradbury, G. Chanan, T. Killeen, Z. M. Lin, N. Gimelshein, L. Antiga, A. Desmaison, A. Köpf, E. Yang, Z. DeVito, M. Raison, A. Tejani, S. Chilamkurthy, B. Steiner, L. Fang, J. J. Bai, S. Chintala. PyTorch: An imperative style, high-performance deep learning library. In Proceedings of the 33rd Conference on Neural Information Processing Systems, Vancouver, Canada, Article No. 721, 2019.
The MONAI Consortium. Project MONAI: Zenodo, [Online, Available: https://doi.org/10.5281/zenodo.4323059, July 28, 2022.
A. Traverso, L. Wee, A. Dekker, R. Gillies. Repeatability and reproducibility of radiomic features: A systematic review. International Journal of Radiation Oncology · Biology · Physics, vol. 102, no. 4, pp. 1143–1158, 2018. DOI: https://doi.org/10.1016/j.ijrobp.2018.05.053.
R. Berenguer, M. del Rosario Pastor-Juan, J. Canales-Vázquez, M. Castro-García, M. V. Villas, F. M. Legorburo, S. Sabater. Radiomics of CT features may be nonreproducible and redundant: Influence of CT acquisition parameters. Radiology, vol. 288, no. 2, pp. 407–415, 2018. DOI: https://doi.org/10.1148/radiol.2018172361.
M. Meyer, J. Ronald, F. Vernuccio, R. C. Nelson, J. C. Ramirez-Giraldo, J. Solomon, B. N. Patel, E. Samei, D. Marin. Reproducibility of CT radiomic features within the same patient: Influence of radiation dose and CT reconstruction settings. Radiology, vol. 293, no. 3, pp. 583–591, 2019. DOI: https://doi.org/10.1148/radiol.2019190928.
S. Zheng, Y. Song, T. Leung, I. Goodfellow. Improving the robustness of deep neural networks via stability training. In Proceedings of IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, USA, pp. 4480–4488, 2016. DOI: https://doi.org/10.1109/CVPR.2016.485.
F. Orlhac, F. Frouin, C. Nioche, N. Ayache, I. Buvat. Validation of a method to compensate multicenter effects affecting CT radiomics. Radiology, vol. 291, no. 1, pp. 53–59, 2019. DOI: https://doi.org/10.1148/radiol.2019182023.
A. Zwanenburg, M. Valliéres, M. A. Abdalah, H. J. W. L. Aerts, V. Andrearczyk, A. Apte, S. Ashrafinia, S. Bakas, R. J. Beukinga, R. Boellaard, M. Bogowicz, L. Boldrini, I. Buvat, G. J. R. Cook, C. Davatzikos, A. Depeursinge, M. C. Desseroit, N. Dinapoli, C. V. Dinh, S. Echegaray, I. E. Naqa, A. Y. Fedorov, R. Gatta, R. J. Gillies, V. Goh, M. Götz, M. Guckenberger, S. M. Ha, M. Hatt, F. Isensee, P. Lambin, S. Leger, R. T. H. Leijenaar, J. Lenkowicz, F. Lippert, A. Losnegaard, K. H. Maier-Hein, O. Morin, H. Müller, S. Napel, C. Nioche, F. Orlhac, S. Pati, E. A. G. Pfaehler, A. Rahmim, A. U. K. Rao, J. Scherer, M. M. Siddique, N. M. Sijtsema, J. S. Fernandez, E. Spezi, R. J. H. M. Steenbakkers, S. Tanadini-Lang, D. Thorwarth, E. G. C. Troost, T. Upadhaya, V. Valentini, L. V. V. Dijk, J. V. Griethuysen, F. H. P. V. Velden, P. Whybra, C. Richter, S. Löck. The Image biomarker standardization initiative: Standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology, vol. 295, no. 2, pp. 328–338, 2020. DOI: https://doi.org/10.1148/radiol.2020191145.
C. Parmar, J. D. Barry, A. Hosny, J. Quackenbush, H. J. W. L. Aerts. Data analysis strategies in medical imaging. Clinical Cancer Research, vol. 24, no. 15, pp. 3492–3499, 2018. DOI: https://doi.org/10.1158/1078-0432.CCR-18-0385.
N. Srivastava, G. Hinton, A. Krizhevsky, I. Sutskever, R. Salakhutdinov. Dropout: A simple way to prevent neural networks from overfitting. Journal of Machine Learning Research, vol. 15, no. 1, pp. 1929–1958, 2014.
J. Koneçný, H. B. McMahan, D. Ramage, P. Richtárik. Federated optimization: Distributed machine learning for on-device intelligence. [Online], Available: http://arxiv.org/abs/1610.02527, March 18, 2022.
Q. Yang, Y. Liu, T. J. Chen, Y. X. Tong. Federated machine learning: Concept and applications. ACM Transactions on Intelligent Systems and Technology, vol. 10, no. 2, Article number 12, 2019. DOI: https://doi.org/10.1145/3298981.
J. Pearl. Causal inference. In Proceedings of Workshop on Causality: Objectives and Assessment, Whistler, Canada, pp. 39–58, 2010.
D. C. Castro, I. Walker, B. Glocker. Causality matters in medical imaging. Nature Communications, vol. 11, no. 1, Article number 3673, 2020. DOI: https://doi.org/10.1038/s41467-020-17478-w.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 61721003) and the Tsinghua-Fuzhou Institute of Data Technologies, China (No. TFIDT 2021003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declared that they have no conflicts of interest to this work.
Additional information
Colored figures are available in the online version at https://link.springer.com/journal/11633
Jiaqi Li received the B. Eng. degree in automation from Beihang University, China in 2018. He is currently a Ph. D. degree candidate in bioinformatics at Department of Automation, Tsinghua University, China.
His research interests include machine learning and its application for medical image analysis.
Zhuofeng Li received the B. Eng. degree in automation from Tsinghua University, China in 2018. He is currently a master student in automation, Tsinghua University, China.
His research interests include machine learning and medical image analysis.
Lei Wei received the B. Eng. and Ph. D. degrees in automation from Tsinghua University, China in 2013 and 2019, respectively. He worked at Department of Automation, Tsinghua University as a postdoctoral fellow from 2019 to 2021, and then joint Beijing National Research Center for Information Science and Technology (BN-RIST), Tsinghua University as an assistant research fellow.
His research interests include systems biology, synthetic biology and single-cell bioinformatics.
Xuegong Zhang received the B. Eng. degree in industrial automation and the Ph. D. degree in pattern recognition and intelligent systems from Tsinghua University, China in 1989 and 1994, respectively. He joined Faculty of Department of Automation, Tsinghua University, China in 1994. From 2001 to 2002, he worked at Harvard School of Public Health as a visiting scientist. He is currently professor of pattern recognition and bioinformatics in Department of Automation, Tsinghua University, and is adjunct professor in School of Life Sciences and School of Medicine, Tsinghua University. He is the director of Bioinformatics Division, Beijing National Research Center for Information Science and Technology (BNRIST). He was elected as the Fellow of the International Society for Computational Biology (ISCB) and of the Chinese Association of Artificial Intelligence (CAAI) in 2020.
His research interests include machine learning methods and their applications in bioinformatics and medical data analysis, including digital twin systems of life, intelligent health and the informatics architecture of human cellular and molecular portraitures.
Rights and permissions
About this article
Cite this article
Li, J., Li, Z., Wei, L. et al. Machine Learning in Lung Cancer Radiomics. Mach. Intell. Res. 20, 753–782 (2023). https://doi.org/10.1007/s11633-022-1364-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11633-022-1364-x