Abstract
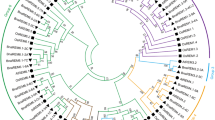
The last step of ethylene biosynthesis in higher plants is to convert 1-aminocyclopropane-1-carboxylic acid (ACC) to ethylene by ACC oxidase (ACO). In our investigation, a cross-introns genomic DNA gene encoding ACC oxidase, PsgACO, was isolated from Paeonia suffruticosa. It revealed that PsgACO (FJ855434) has 1281 bases, containing four exons and three introns, encoding 312 amino acids. The four exons stretched from 1 to 105, 217 to 434, 592 to 925 and 1000 to 1281 bases. A splicing junction sequence of all the exon-introns conformed to the GT-AG rule in the cross-intron genomic DNA sequence of the ACO gene. PsgACO showed high homology to many characterized ACC oxidases both at the nucleic acid and amino acid levels. As well, twelve amino acid residues were conserved among many ACOs from other species. A phylogenetic tree analysis indicated that the amino acid of ACOs is quite conserved among the different eudicots. The phylogenetic tree showed that both the tree peony and herbaceous peony are quite isolated taxa. Bioinformatic analysis showed that the molecular weight of ACO is 35.29 kD, with a theoretical pI of 5.25. It is a non-secrete, stable hydrophilic protein, located in the cytoplasm.
Similar content being viewed by others
References
Atkinson R G, Gunaseelan K, Wang M Y, Luo L, Wang T, Norling C L, Johnston S L, Maddumage R, Schroder R, Schaffer R J. 2011. Dissecting the role of climacteric ethylene in kiwifruit (Actinidia chinensis) ripening using an 1-aminocyclopropane-1-carboxylic acid oxidase knockdown line. J Exp Bot, 62(11): 3821–3825
Beruto M, Lanteri L, Portogallo C. 2004. Micropropagation of tree peony (Paeonia suffruticosa). Plant Cell Tiss Org, 79(2): 249–255
Brown J W S. 1986. A catalogue of splice junctions and putative branch point sequences from plant introns. Nucl Acid Res, 14(24): 9549–9559
Burge C, Karlin S. 1997. Prediction of complete gene structures in human genomic DNA. J Mol Biol, 268(1): 78–94
Burge C B, Karlin S. 1998. Finding the genes in genomic DNA. Curr Opin Struc Biol, 8(3): 346–354
Cheng F Y. 2007. Advances in the breeding of tree peonies and a cultivar system for the cultivar group. Intl J Plant Breed, 1(2): 89–104
Doyle J J, Doyle J L. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull, 9: 1–15
Guo W W, Dong L, Wang L Y, Chen R X, Liu A Q. 2004. The postharvest characteristics and water balance of some cultivars of tree-peony cut flowers. Sci Silv Sin, 40(4): 89–93 (in Chinese with English abstract)
Lasserre E, Bouquin T, Hernandez J A, Bull J, Pech J C, Balague C. 1996. Structure and expression of three genes encoding ACC oxidase homologs from melon (Cucumis melo L). Mol Gen Genet, 251(1): 81–90
Li J J. 2005. Chinese Tree Peony: Xibei, Xinan, Jiangnan volume. Beijing: China Forestry Publishing House
Mbeguie-A-Mbeguie D, Chahine H, Gomez R M, Gouble B, Reich M, Audergon J M, Souty M, Albagnac G, Fils-Lycaon B. 1999. Molecular cloning and expression of a cDNA encoding 1-aminocyclopropane-1-carboxylate (ACC) oxidase from apricot fruit (Prunus armeniaca). Phys Plant, 105(2): 294–303
Mcgarvey D J, Christoffersen R E. 1992. Characterization and kinetic-parameters of ethylene-forming enzyme from avocado fruit. J Biol Chem, 267(9): 5964–5967
Reddy A S. 2007. Alternative splicing of pre-messenger RNAs in plants in the genomic era. Annu Rev Plant Biol, 58: 267–294
Shi G A, Guo X F, Han J G, Sun X M, Yang Z S. 1999. A study on ethylene production and lipid peroxidization in florescence and flower senescence of Paeonia suffruticosa. J Northwest Sci-tech Univ Agricult Forest, 27(5): 50–53 (in Chinese with English abstract)
Smith J J, Ververidis P, John P. 1992. Characterization of the ethylene-forming enzyme partially purified from melon. Phytochemistry, 31(5): 1485–1494
Stern F C. 1946. A Study of the Genus Paeonia. London: The Royal Horticultural Society
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol, 25(7): 1253–1256
Tamura M. 1997. Taxonomic studies of the Ranunculacea: retrospect and prospect. Mem School, B. O. S. T. Kinki University, 2: 69–85
Tang X Y, Wang H, Brandt A S, Woodson W R. 1993. Organization and structure of the 1-aminocyclopropane-1-carboxylate oxidase gene family from Petunia Hybrida. Plant Mol Biol, 23(6): 1151–1164
Wang R H, Liu Y L, Li J R. 2005. Studies on the blossom physiology in the different development stage of peony and Chinese peony flower. Acta Horticult Sin, 32(5): 861–865 (in Chinese with English abstract)
Xiao P G, He C N, Peng Y, Zhang Y C, Xu L J, Gu J. 2010. Phytochemical and biological studies of Paeoniaceae. Chem Biodivers, 7(4): 805–838
Xu X D, Chen G C, Bai Y M, Yang Z S, Chen H B. 1987. A study on the effects of chemicals on the vase-life of cut peony flowers. Acta Horticult Sin, 14(1): 69–72 (in Chinese with English abstract)
Yang S F, Hoffman N E. 1984. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Phys, 35: 155–189
Zhou L, Dong L. 2008. Cloning and sequence analysis of 1-amino cyclopropane-1-carboxylic acid oxidase gene cDNA from tree peony. Acta Horticult Sin, 35(6): 891–894 (in Chinese with English abstract)
Zhou L, Dong L, Jia P Y, Wang W R, Wang L Y. 2010. Expression of ethylene receptor and transcription factor genes, and ethylene response during flower opening in tree peony (Paeonia suffruticosa). Plant Growth Regul, 62(2): 171–179
Zhou L, Jia P Y, Liu J, Wang W R, Huo Z P, Dong L. 2009. Effect of ethylene on cut flowers of tree peony ‘Luoyang Hong’ opening and senescence process and endogenous ethylene biosynthesis. Acta Horticult Sin, 36(3): 239–244 (in Chinese with Engli sh abstract)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, By., Gao, Sp., Hou, Xg. et al. Cloning and analysis of cross-intron genomic gene encoding ACO in Paeonia suffruticosa Andr.. For. Stud. China 14, 291–298 (2012). https://doi.org/10.1007/s11632-012-0403-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11632-012-0403-z