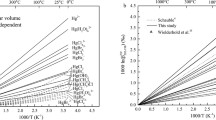
Abstract
It is well-known that the equilibrium isotope fractionation of mercury (Hg) includes classical mass-dependent fractionations (MDFs) and nuclear volume effect (NVE) induced mass-independent fractionations (MIFs). However, the effect of the NVE on these kinetic processes is not known. The total fractionations (MDFs + NVE-induced MIFs) of several representative Hg-incorporated substances were selected and calculated with ab initio calculations in this work for both equilibrium and kinetic processes. NVE-induced MIFs were calculated with scaled contact electron densities at the nucleus through systematic evaluations of their accuracy and errors using the Gaussian09 and DIRAC19 packages (named the electron density scaling method). Additionally, the NVE-induced kinetic isotope effect (KIE) of Hg isotopes are also calculated with this method for several representative Hg oxidation reactions by chlorine species. Total KIEs for 202Hg/198Hg ranging from − 2.27‰ to 0.96‰ are obtained. Three anomalous 202Hg-enriched KIEs (δ202Hg/198Hg = 0.83‰, 0.94‰, and 0.96‰,) caused by the NVE are observed, which are quite different from the classical view (i.e., light isotopes react faster than the heavy ones). The electron density scaling method we developed in this study can provide an easier way to calculate the NVE-induced KIEs for heavy isotopes and serve to better understand the fractionation mechanisms of mercury isotope systems.










Similar content being viewed by others
Data availability
All data and models used during the study appear in the submitted article.
Code availability
N/A.
References
Almoukhalalati A, Shee A, Saue T (2016) Nuclear size effects in vibrational spectra. Phys Chem Chem Phys. 18:15406–15417.
Angeli I (2004) A consistent set of nuclear rms charge radii: Properties of the radius surface R(N, Z). Atom Data Nucl Data Tables. 87:185–206.
Angeli I, Marinova KP (2013) Table of experimental nuclear ground state charge radii: An update. Atom Data Nucl Data Tables. 99(1):69–95.
Aufmuth P, Heilig K, Steudel A (1987) Changes in mean-square nuclear charge radii from optical isotope shifts. Atom Data Nucl Data Tables. 37:455–490.
Barysz M, Sadlej AJ (2001) Two-component methods of relativistic quantum chemistry: from the Douglas-Kroll approximation to the exact two-component formalism. J Mol Struct. 573:181–200.
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys. 98:5648–5652.
Bergquist BA, Blum JD (2007) Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science. 318:417–420.
Bergquist BA, Blum JD (2009) The odds and evens of mercury isotopes: Applications of mass-dependent and mass-independent isotope fractionation. Elements. 5(6):53–357.
Bigeleisen J (1996) Nuclear size and shape effects in chemical reactions. Isotope chemistry of the heavy elements. J Am Chem Soc. 118:3676–3680.
Bigeleisen J, Mayer MG (1947) Calculation of equilibrium constants for isotopic exchange reactions. J Chem Phys. 15:261–267.
Bigeleisen J, Wolfsberg M (1958) Theoretical and experimental aspects of isotope effects in chemical kinetics. Adv Chem Phys. 1:15–76.
Blum JD (2011) Applications of stable mercury isotopes to biogeochemistry. Handbook of environmental isotope geochemistry, Vol 15. Springer, Cham, pp 229–245.
Blum JD, Johnson MW (2017) Recent developments in mercury stable isotope analysis. Rev Min Geochem. 82(1):733–757.
Blum JD, Sherman LS, Johnson MW (2014) Mercury isotopes in Earth and environmental sciences. Ann Rev Earth Planet Sci. 42(1):249–269.
Chen JB, Hintelmann H, Feng XB, Dimock B (2012) Unusual fractionation of both odd and even mercury isotopes in precipitation from Peterborough, ON, Canada. Geochim Cosmochim Acta. 90:33–46.
Dunning TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys. 90:1007–1023.
Dyall KG (2023) Dyall double-zeta, triple-zeta, and quadruple-zeta basis set archive files. Zenodo. https://doi.org/10.5281/zenodo.7606547
Dzurko M, Foucher D, Hintelmann H (2009) Determination of compound–specific Hg isotope ratios from transient signals using gas chromatography coupled to multicollector inductively coupled plasma mass spectrometry (MC-ICP/MS). Anal Bioanal Chem. 393(1):345–355.
Estrade N, Carignan J, Sonke JE, Donard OFX (2009) Mercury isotope fractionation during liquid–vapor evaporation experiments. Geochim Cosmochim Acta. 73:2693–2711.
Eyring H (1935) The activated complex in chemical reactions. J Chem Phys. 3:107–115.
Fang T, Liu Y (2019) Equilibrium thallium isotope fractionation and its constraint on Earth’s late veneer. Acta Geochim. 38(4):459–471.
Franzke YJ, Spiske L, Pollak P, Weigend F (2020) Segmented contracted error-consistent basis sets of quadruple-ζ valence quality for one- and two-component relativistic all- electron calculations. J Chem Theory Comput. 16:5658–5674.
Fricke G, Heilig K (2004) 80-Hg mercury. Numerical data and functional relationships in science and technology. Group I: Element particles, nuclei and atoms. Nuclear charge radii, Vol 20. Springer, Heidelberg, pp 1–9.
Frisch MJ et al (2009) Gaussian software package, Gaussian09, Revision D.01. Inc, Wallingford CT.
Fu SL, Hu RZ, Yin RS, Yan J, Mi XF, Song ZC, Sullivan NA (2020) Mercury and in-situ sulfur isotopes as constraints on the metal and sulfur sources for the world’s largest Sb deposit at Xikuangshan, southern China. Min Depos. 55:1353–1364.
Fujii T, Moynier F, Albarède F (2009) The nuclear field shift effect in chemical exchange reactions. Chem Geol. 267:139–156.
Ghosh S, Schauble EA, Couloume GL, Blum JD, Bergquist BA (2013) Estimation of nuclear volume dependent fractionation of mercury isotopes in equilibrium liquid-vapor evaporation experiments. Chem Geol. 336:5–12.
Gomes ASP et al (2019) DIRAC a relativistic ab initio electronic structure program, Release DIRAC19. https://doi.org/10.5281/zenodo.3572669
Grimme S (2006) Semiempirical hybrid density functional with perturbative second-order correlation. J Chem Phys. 124:1–16.
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J Chem Phys. 132:1–19.
King WH (1984) Isotope shifts in atomic spectra. Plenum Press, New York.
Li LC, Deng P, Tian AM, Xu MH, Zheng CG, Wong NB (2003) A study on the reaction mechanism and kinetic of mercury oxidation by chlorine species. J Mol Struct. (Theochem) 625:277–281.
Li ZG, Feng XB, He TR, Yan HY, Liang L (2005) Determination of total mercury in soil and sediment by aquaregia digestion in the water bath coupled with cold vapor atom fluorescence spectrometry. Bull Min Pet Geochem. 24(2):140–143.
Li SL, Li LH, Lan YC, Lv BY (2016) Rapid determination of total mercury in sediment by using Lumex analytical equipment. Guangzhou Chem. 41(2):68–71.
Lin HL, Zhu RL, Yu L, Cheng YX, Zhu RR, Liu P, Ren ZH (2020) Determination of arsenic, mercury, selenium, antimony and bismuth in soil and sediments by water bath digestion–atomic fluorescence spectrometry. Spectrosc Spect Anal. 40(5):1528–1533.
Liu YF, Qi HW, Bi XW, Hu RZ, Qi LK, Yin RS, Tang YY (2021) Mercury and sulfur isotopic composition of sulfides from sediment–hosted lead–zinc deposits in Lanping basin Southwestern China. Chem Geol. 559:119910.
Lu T, Chen F (2012) Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem. 33:580–592.
Pantazis DA, Chen XY, Landis CR, Neese F (2008) All-electron scalar relativistic basis sets for third-row transition metal atoms. J Chem Theory Comput. 4(6):908–919.
Peterson KA, Puzzarini C (2005) Systematically convergent basis sets for transition metals. II. Pseudopotential-based correlation consistent basis sets for the group 11 (Cu, Ag, Au) and 12 (Zn, Cd, Hg) elements. Theor Chem Acc. 114:283–296.
Redlich O (1935) Eine allgemeine beziehung zwischen den schwingungsfrequenzen isotoper molekeln. Z Phys Chem B. 28:371–382 (in German).
Schauble EA (2007) Role of nuclear volume in driving equilibrium stable isotope fractionation of mercury, thallium, and other very heavy elements. Geochim Cosmochim Acta. 71:2170–2189.
Schauble EA (2013) Modeling nuclear volume isotope effects in crystals. Proc Natl Acad Sci USA. 110:17714–17719.
Sherman LS, Blum JD, Johnson KP, Keeler GJ, Barres JA, Douglas TA (2010) Mass-independent fractionation of mercury isotopes in arctic snow driven by sunlight. Nat Geosci. 3:173–177.
Sial AN, Lacerda LD, Ferreira VP, Frei R, Marquillas RA, Barbosa JA, Gaucher C, Windmöller CC, Pereira NS (2013) Mercury as a proxy for volcanic activity during extreme environmental turnover: The Cretaceous-Paleogene transition. Palaeogeogr Palaeoclimatol Palaeoecol. 387(7):153–164.
Smith CN, Kesler SE, Klaue B, Blum JD (2005) Mercury isotope fractionation in fossil hydrothermal systems. Geology. 33(10):825–828.
Stacey DN (1966) Isotope shifts and nuclear charge distributions. Rep Prog Phys. 29:171–215.
Sun RY, Sonke JE, Liu GJ (2016) Biogeochemical controls on mercury stable isotope compositions of world coal deposits: A review. Earth Sci Rev. 152:1–13.
Sun T, Gaut A, Tang S, Huang YX, ElSherief M, Zhao JY, Mirza D, Belding E, Chang KW, Wang WY (2019) Mitigating gender bias in natural language processing: Literature review. Comput Linguist Assoc. 57:1630–1640.
Thibodeau AM, Ritterbush K, Yager JA, Joshua West A, Ibarra Y, Bottjer DJ, Berelson WM, Bergquist BA, Corsetti FA (2016) Mercury anomalies and the timing of biotic recovery following the end-Triassic mass extinction. Nat Commun. 7:1–8.
Urey HC (1947) The thermodynamic properties of isotopic substances. J Chem Soc. https://doi.org/10.1039/jr9470000562
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys Chem Chem Phys. 7(18):3297–3305.
Wilson AK, Woon DE, Peterson KA, Dunning TH Jr (1999) Gaussian basis sets for use in correlated molecular calculations. IX. the atoms gallium through krypton. J Chem Phys. 110:7667–7676.
Xu CX, Yin RS, Peng JT, Hurley JP, Lepak RF, Gao JF, Feng XB, Hu RZ, Bi XW (2018) Mercury isotope constraints on the source for sediment–hosted lead–zinc deposits in the Changdu Area, southwestern China. Miner Deposita. 53:339–352.
Xu CX, Meng YM, Huang C, Tang C, Zheng FW (2021) Advances in the study on mercury isotope geochemistry and its application in mineral deposits. Rock Miner Anal. 40(2):173–186.
Yang S, Liu Y (2015) Nuclear volume effects in equilibrium stable isotope fractionations of mercury, thallium and lead. Sci Rep. 5:1–10.
Yang S, Liu Y (2016) Nuclear field shift effects on stable isotope fractionation: A review. Acta Geochim. 35(3):227–239.
Yin RS, Feng XB, Chen J (2014) Mercury stable isotopic compositions in coals from major coal producing fields in China and their geochemical and environmental implications. Environ Sci Technol. 48(10):5565–5574.
Zhu CW, Tao CH, Yin RS, Liao SL, Yang WF, Liu J, Barriga FJAS (2020) Seawater versus mantle sources of mercury in sulfide–rich seafloor hydrothermal systems, southwest Indian Ridge. Geochim Cosmochim Acta. 281:91–101.
Acknowledgements
This paper is supported by Chinese NSF project (42130114), the strategic priority research program (B) of CAS (XDB41000000) and the pre-research Project on Civil Aerospace Technologies No. D020202 funded by Chinese National Space Administration (CNSA)
Funding
Chinese NSF project (42130114), the strategic priority research program (B) of CAS (XDB41000000) and the pre-research Project on Civil Aerospace Technologies (No. D020202) funded by Chinese National Space Administration (CNSA).
Author information
Authors and Affiliations
Contributions
Yining Zhang and Yun Liu selected topics; Yining Zhang conceived and designed methods; Chenlu Yang calculated data, analyzed and compiled the first draft; Yining Zhang and Yun Liu reviewed and revised the first draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
N/A.
Consent to participate
N/A.
Consent for publication
N/A.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, C., Zhang, Y. & Liu, Y. Nuclear volume effects in kinetic isotope fractionation: A case study of mercury oxidation by chlorine species. Acta Geochim (2024). https://doi.org/10.1007/s11631-024-00691-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11631-024-00691-5