Abstract
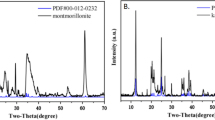
Adsorption experiments were made at room temperature and neutral pH value on different types of minerals associated with the Lower Cambrian black shale series polymetallic layers in Hunan and Guizhou provinces on nanometer-sized Pt colloids and PtCl4 2−-bearing ionic solutions with an attempt to constrain the relationship between the different types of minerals in the polymetallic layers and the enrichment of platinum group elements (PGEs). Experimental results showed that the different types of minerals show strong selectivity to the adsorption of nanometer-sized Pt colloids and PtCl4 2−-bearing ionic solutions. Metallic sulfides, organic matter and clay minerals are the strong adsorbents of PGEs, while quartz, albite, muscovite and other silicate minerals show a week adsorbility to both of them. This phenomenon is well consistent with the geological fact that metallic sulfides, organic matter and clay minerals in the polymetallic layers of the black shale series are the major carrier minerals of PGEs, giving a thorough explanation to the mechanism of enrichment of previous metal elements. Adsorption may be a principal mechanism of enrichment of precious metal elements under lower temperature conditions. The presence of the aforementioned strong adsorbents is the good geochemical barriers for the enrichment of PGEs.
Similar content being viewed by others
References
Ahmadi T.S., Wang Z.L., Green T.C., Henglein A., and Mostafa A. (1996) Shape-controlled synthesis of colloidal platinum nanoparticles [J]. Science. 272, 1924–1926.
Bancroft G.M. and Jean G. (1982) Gold deposition at low temperature on sulfide minerals [J]. Nature. 298, 730–731.
Chen Nansheng, Yang Xiuzhen, Liu Dehan, Xiao Xuejun, Fan Delian, and Wang Lianfang (1990) Lower Cambrian Black Rcok Series and associated stratiform deposits in Southern China [J]. Chinese Journal of Geochemistry. 8, 244–255.
Distler V.V., Yudovskaya M.A., Mitrofanov G.L., Prokof’ev V.Y., Lishnevskii E.N. et al. (2004) Geology, composition, and genesis of the Sukhoi Log noble metals deposit, Russia [J]. Ore Geology Reviews. 24, 7–44.
Horan M.F., Morgan J.F., Grauch R.I., Coveney R.M., Murowchick J.B., and Hulbert L.J. (1994) Rhenium and osmium isotopes in black shales and Ni-Mo-PGE-rich sulfide layers, Yukon-Territory, Canada, and Hunan and Guizhou provinces, China [J]. Geochimica et Cosmochimica Acta. 58, 257–265.
Jia Jianye, Zhu Zizun, and Lan Binming (1996) Establishment of the adsorption-reduction-growth model of gold in pyrite [J]. Northwest Geology. 17, 33–37 (in Chinese with English abstract).
Jiang Yonghong and Li Shengrong (2005) Study on the isotope data tracing and isotopic chronology in the black-rock-series-type Ni-Mo deposites in the Lower Cambrian in Hunan and Guizhou Provinces [J]. Mineral and Rocks. 25, 62–66 (in Chinese with English abstract).
Jiang Zechun, Mo Deming, and Chen Damei (1999) Experiment on the adsorption of gold on polar minerals [J]. Acta Mineralogica Sinica. 19, 356–361 (in Chinese with English abstract).
Kucha H. (1982) Platinum-group metals in the Zechstein copper-deposits, Poland [J]. Economic Geology. 77, 1578–1591.
Li Shengrong (1994) Study on the Geochemistry of Gold, Silver and PGEs in the Lower Cambrian Black Rocks of Hunan-Guizhou Region [D]. Post-doctor Dissertation of Institute of Geochemistry, Chinese Academy of Sciences (in Chinese with English abstract).
Li Shengrong, Xiao Qiyun, Shen Junfeng, Sun Li, Liu Bo, Yan Baikun, and Jiang Yonghong (2003) Rhenium-osmium isotope constraints on the age and source of the platinum mineralization in the Lower Cambrian black rock series of Hunan-Guizhou provinces, China [J]. Science in China (Series D, Earth Sciences). 46, 919–927.
Liu Yingjun, Cao Liming, Li Zhaolin, Wang Henian, Chu Tongqing, and Zhang Jingrong (1984) Geochemistry of the Elements [M]. pp.1–547. Science Press, Beijing (in Chinese).
Mao Jingwen, Bernd Lehmann, Du Andao., Zhang Guangdi., Ma Dongsheng, Wang Yitian., Zeng Mingguo, and Robert Kerrich (2002) Re-Os dating of polymetallic Ni-Mo-PGE-Au mineralization in lower Cambrian black shales of South China and its geologic significance [J]. Economic Geology and the Bulletin of the Society of Economic Geologists. 97, 1051–1061.
Tu Guangchi (1998) Low Temperature Geochemistry [M]. pp.76–92. Science Press, Beijing (in Chinese).
Wang Min, Sun Xiaoming, and Ma Mingyang (2005) Microthermometric measurement of fluid inclusions and its constraints on genesis of PGE-polymetallic deposits in Lower Cambrian black rock series, southern China [J]. Chinese Journal of Geochemistry. 24, 297–305.
Wang Min and Sun Xiaoming (2007) Geology, Geochemistry and Genesis of PGE-polymetallic Deposit in Black Rock Series, Sourth China [M]. pp.1–136. Geological Publishing House, Beijing (in Chinese).
Wang Zhonggang, Yu Xueyuan, and Zhao Zhenhua (1989) REE Geochemistry [M]. pp.321–342. Science Press, Beijing (in Chinese).
Wood S.A., Mountain B.W., and Fenlon B.J. (1989) Thermodynamic constraints on the solubility of platinum and palladium in hydrothermal solutions: Reassessment of hydroxide, bisulfide, and ammonia complexing [J]. Economic Geology. 84, 2020–2028.
Wu Daqing, Diao Guiyi, Wei Junfeng, and Yuan Peng (2000) Surface function groups and surface reactions of minerals [J]. Geological Journal of Chinese Universities. 6, 225–232 (in Chinese with English abstract).
Wu Daqing, Peng Jinlian, and Cheng Guoxi (1996) Adsorption of silver ions onto sulfide minerals and its significance in mineralization [J]. Geochimica. 25, 372–378 (in Chinese with English abstract).
Wu Honghai and Wu Daqing (1998a) Research progress on reaction between trace elements and minerals surface [J]. Bulletin of Mineralogy, Petrology and Geochemistry. 17, 191–196 (in Chinese).
Wu Honghai, Wu Daqing, and Pen Jinlian (1998b) Experimental study on surface reactions of heavy metal ions with quartz [J]. Geochimica. 27, 523–531 (in Chinese with English abstract).
Zhu Xiaoqing, Huang Yan, Zhang Qian, and He Yuliang (2005a) Experimental study on selective adsorption behaviors of silver and gold, and its significance [J]. Mineral Deposits. 24, 445–450 (in Chinese with English abstract).
Zhu Xiaoqing and Wang Zhonggang (2005b) Advances in the study of nano-sized materials [J]. Progress in Natural Science. 15, 385–389 (in Chinese).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11631-011-0542-2
Rights and permissions
About this article
Cite this article
Han, T., Zhu, X. & Li, Z. Simulating experiment on the enrichment of precious metals in Lower Cambrian black shale series of Hunan and Guizhou provinces. Chin. J. Geochem. 30, 375–381 (2011). https://doi.org/10.1007/s11631-011-0522-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-011-0522-6