Abstract
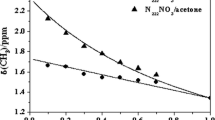
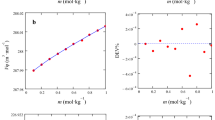
Molecular dynamics simulation method was used to study the influence of \(NO_2^ - \) on both the structure and properties of the binary nitrate salts (60 wt.% NaNO3 + 40 wt.% KNO3). The density and viscosity of the mixtures were experimentally measured and the simulation results met well with the experimental ones. The simulation results showed that, with the addition of \(NO_2^ - \), the ionic clusters tended to loose and the mobilities of all the ions tended to increase. The density, viscosity, and heat capacity decreased while the thermal conductivity increased with the increase of +++ concentration. The correlation between the microscopic structure and physical properties of the mixtures were discussed and revealed.
Similar content being viewed by others
References
Fernández A.G., Ushak S., Galleguillos H., et al., Development of new molten salts with LiNO3 and Ca(NO3)2 for energy storage in CSP plants. Applied Energy, 2014, 119: 131–140.
Peng Q., Yang X., Ding J., et al., Design of new molten salt thermal energy storage material for solar thermal power plant. Applied Energy, 2013, 112: 682–689.
Wang T., Mantha D., Reddy R.G., Novel low melting point quaternary eutectic system for solar thermal energy storage. Applied Energy, 2013, 102: 1422–1429.
Cordaro J.G., Rubin N.C., Bradshaw R.W., Multicomponent molten salt mixtures based on nitrate/ nitrite anions. Journal of Solar Energy Engineering, 2011, 133(1): 011014.
Bradshaw R.W., Cordaro J.G., Siegel N.P., Molten nitrate salt development for thermal energy storage in parabolic trough solar power systems. ASME 2008 2nd International Conference on Energy Sustainability collocated with the Heat Transfer, Fluids Engineering, and 3rd Energy Nanotechnology Conferences. Jacksonville, Florida, USA, 2008, ASME 2008 2nd International Conference on Energy Sustainability, Volume 2, paper No. ES2008-54174, pp. 631–637.
Yamaguchi T., Okada I., Ohtaki H., et al., X-ray and neutron diffraction and molecular dynamics simulation of molten lithium and rubidium nitrates. Molecular Physics, 1986, 58(2): 349–364.
Adya A.K., Takagi R., Kawamura K., et al., Structural determination of molten NaNO3, NaNO2 and their eutectic mixture by molecular dynamics simulation and X-ray diffraction. Molecular Physics, 1987, 62(1): 227–238.
Kato T., Machida K., Oobatake M., et al., Vibrational dephasing in computer simulated molten LiNO3. The Journal of Chemical Physics, 1990, 93(6): 3970.
Kato T., Machida K., Oobatake M., et al., Ionic dynamics in computer simulated molten LiNO3. III. Effect of the potential well on the translational and reorientational motions. The Journal of Chemical Physics, 1990, 92(9): 5506.
Kato T., Machida K., Oobatake M., et al., Ionic dynamics in computer simulated molten LiNO3. I. Translational and reorientational motion. The Journal of Chemical Physics, 1988, 89(5): 3211.
Kato T., Machida K., Oobatake M., et al., Ionic dynamics in computer simulated molten LiNO3. II. Tumbling and spinning motions of nitrate ions. The Journal of Chemical Physics, 1988, 89(12): 7471.
Ribeiro M.C.C., On the Chemla effect in molten alkali nitrates. The Journal of Chemical Physics, 2002, 117(1): 266.
Jayaraman S., Thompson A.P, von Lilienfeld O.A., et al., Molecular simulation of the thermal and transport properties of three alkali nitrate salts. Industrial & Engineering Chemistry Research, 2010, 49(2): 559–571.
Ni H., Wu J., Sun Z., et al., Molecular simulation of the structure and physical properties of alkali nitrate salts for thermal energy storage. Renewable Energy, 2019, 136: 955–967.
Peng Q., Ding J., Wei X., et al., The preparation and properties of multi-component molten salts. Applied Energy, 2010, 87(9): 2812–2817.
Peng Q., Wei X., Ding J., et al., High-temperature thermal stability of molten salt materials. International Journal of Energy Research, 2008, 32(12): 1164–1174.
Hu X., Yu Z., Gao B., et al., Equilibrium between \(NO_3 - \) and \(NO_2 - \) in KNO3-NaNO2 melts: a Raman spectra study. Chinese Optics Letters, 2014, 12(9): 96–100.
Hu X., Li Y., Yu Z., et al., Thermal stability of sodium nitrate-sodium nitrite melts: A Raman spectra study. Spectroscopy Letters, 2018, 51(7): 350–355.
Lynden-Bell R.M., Impey R.W., Klein M.L., Investigation of the lattice vibrations of solid NaNO2 by means of molecular dynamics calculations. Chemical physics, 1986, 109(1): 25–33.
Janz G.J., Mikawa Y., The evaluation of Urey-Bradley force constants in planar XY3 type molecules. Journal of Molecular Spectroscopy, 1960, 1: 92–100.
Hisatsune I.C., Devlin J.P, Califano S., Urey-Bradley potential constants in nitrogen dioxide, nitrite and dinitrogen tetroxide. Spectrochimica Acta, 1960, 16: 450–458.
Plimpton S., Fast parallel algorithms for short-range molecular dynamics. Journal of Computational Physics, 1995, 117(1): 1–19.
Browna W.M., Wang P., Plimpton S.J., et al., Implementing molecular dynamics on hybrid high performance computers - short range forces. Computer Physics Communications, 2011, 182: 898–911.
Cheng J., Zhang P., An X., et al., A device for measuring the density and liquidus temperature of molten fluorides for heat transfer and storage. Chinese Physics Letters, 2013, 30(12): 126501.
Sun Z., Hu C., Ni H., et al., Influence of impurity \(NO_4 - \) on the thermal performance of molten nitrates used for thermal energy storage. Energy Technology, 2018, 10(6): 2065–2073.
Pauvert O., Salanne M., Zanghi D., et al., Ion specific effects on the structure of molten AF-ZrF4 systems (A+= Li+, Na+, and K+). The Journal of Physical Chemistry B, 2011, 115(29): 9160–9167.
Levesque M., Sarou-Kanian V, Salanne M., et al., Structure and dynamics in yttrium-based molten rare earth alkali fluorides. The Journal of Chemical Physics, 2013, 138(18): 184503.
Corradini D., Madden P.A., Salanne M., Coordination numbers and physical properties in molten salts and their mixtures. Faraday Discuss, 2016, 190: 471–486.
Duncan A., Introduction to chemical engineering processes. Global Media, Delhi, 2009.
Wang J., Sun Z., Lu G, et al., Molecular dynamics simulations of the local structures and transport coefficients of molten alkali chlorides. The Journal of Physical Chemistry B, 2014, 118(34): 10196–10206.
Wang J., Wu J., Sun Z., et al., Molecular dynamics study of the transport properties and local structures of molten binary systems (Li, Na)Cl, (Li, K)Cl and (Na, K)Cl. Journal of Molecular Liquids, 2015, 209: 498–507.
Alder B.J., Wainwright T.E., Studies in molecular dynamics. II. Behavior of a small number of elastic spheres. The Journal of Chemical Physics, 1960, 33(5): 1439–1451.
Muller-Plathe F., Reversing the perturbation in nonequilibrium molecular dynamics: An easy way to calculate the shear viscosity of fluids. Physical Review E., 1999, 59(5): 4894–4898.
Bordat P., Müller-Plathe F., The shear viscosity of molecular fluids: A calculation by reverse nonequilibrium molecular dynamics. The Journal of Chemical Physics, 2002, 116(8): 3362–3369.
Ding J., Pan G., Du L., et al., Molecular dynamics simulations of the local structures and transport properties of Na2CO3 and K2CO3. Applied Energy, 2018, 227: 555–563.
Tenney C.M., Maginn E.J., Limitations and recommendations for the calculation of shear viscosity using reverse nonequilibrium molecular dynamics. The Journal of Chemical Physics, 2010, 132(1): 14103.
Ni H., Wu J., Sun Z., et al., Insight into the viscosity enhancement ability of Ca(NO3)2 on the binary molten nitrate salt: A molecular dynamics simulation study. Chemical Engineering Journal, 2019, 377: 120029.
Acknowledgments
This project is supported by Grant 51774144 and U1407126 from the National Natural Science Foundation of China, and Grant 2018YFC1903803 from the National Key R&D Program of China. The following local government funding also supported this project: Grant 2017-ZJ-727 and 2018-ZJ-738 from Applied basic research of Qinghai Province, Grant 2015-GX-Q19A from Capacity Building of Qinghai Engineering Research Center for Integrated Utilization of Salt Lake and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ni, H., Wu, J., Sun, Z. et al. Influence of \(NO_2^ - \) on the Microscopic Structure and Physical Properties of the Binary Nitrate Salts: a Molecular Dynamics Simulation Study. J. Therm. Sci. 29, 464–476 (2020). https://doi.org/10.1007/s11630-020-1226-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11630-020-1226-1