Abstract
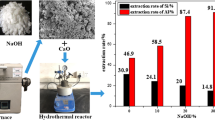
Coal gangue was calcinated under air, nitrogen, carbon dioxide, air–hydrogen, and hydrogen atmospheres. The effects of different calcination temperatures and atmospheres on the mineral composition of activated coal gangue were investigated by X-ray diffraction. Moreover, the acid leaching kinetics of aluminum oxide from coal gangue was investigated with sulfuric acid. It showed that the air atmosphere promoted kaolinite decomposition during coal gangue calcination. The hydrogen atmosphere promoted the activation and decomposition of kaolinite at reaction temperatures exceeding 650°C. The carbon dioxide atmosphere eliminated the influence of residual carbon on coal gangue. When the ratio of acid/coal gangue was 1.5 and reaction temperature was 650°C, the sulfuric acid leaching rate under air, air-hydrogen, carbon dioxide, hydrogen and nitrogen atmospheres were 93.66%, 90.90%, 84.06%, 81.91% and 77.54% respectively. The acid leaching reaction process conformed to unreacted shrinking core model of particle unchanged, and was controlled by the interfacial chemical reaction. The reaction kinetic equation for the leaching process was 1-(1-x)1/3=kt with an apparent activation energy of 48.97 kJ/mol.
Similar content being viewed by others
References
Zhou C., Chang X., Survey of the Technology of Comprehensive Utilization of Coal Gangue. Coal Preparatio. Technology, 2007, (4): p.2–3.
Jiang A., The Composition Characteristics of Gangue and the Utilization Way. Chin. Coal, 2000, 26(3): p.25–27.
Leng F., The Comprehensive Utilization of Coal Slack. Building Science Researcho. Sichuan, 2000, 26(2): p.44–46.
Gu L., Xia J. and Zhang Z., Affecting Factors on the Alumina Extracting from the Coal Gangue with Sulfuric Acid. Journal of Safety an. Environment, 2012, 12(2): p.88–91.
Zhang J., Tong J. and Sun Pe., Study on Sintering Process of Raw Materials in Extracting Alumina From Coal Gangue. Hydrometallurgy o. China, 2011, 30(4): p.316–319.
Si P., Activaation Technology for Aluminum Recovery from Coal Spoil through Acid Leaching Route. Shanghai: East China University of Science and Technology, 2011.
Gong C., Song X. and Li D., Mechanism Discussion on Calcinated Activate Coal Gangue. Journal of Materials Science. Engineering, 2005, 23(1): p.1–2.
Li Y., Wang W. and Yang X., Analysis of Thermal Activation and Phase Transformation of Coal-gangue. Journal of the Chinese Cerami. Society, 2007, 35 (9): p.1259–1260.
Li Y., Wang W. and Yang X., Thermal Activation and Influential Factors of Coal-gangue. Coa. Conversion, 2007, 30(1): p.1–2.
Guo J., Kaolinite-Mullite Reaction Series: A 27Al and 29Si MASNMR Study. Acta Mineralogic. Sinica, 1997, 17(3): p.250–260.
Wei C., Study on Phase Transformation of Calcinated Coal Kaolinite and Activity of Volcanic Ash. Bulletin of the Chinese Cerami. Society, 2005, 24(2): p.13–16.
Wei C., Influence of Temperature on Phase Transformation of Calcinated Kaolinite and Si, Al Activity. Acta Mineralogica Sinica, 2005, 25(3): p.197–200.
Zheng S., Li Y. and Xu X., Study of Effects of Temperature on the Physical and Chemical Properties of Calcinated Kaolinite. Journal of the Chinese Cerami. Society, 2003, 31(4): p.417–420.
Zhang Z. and Zhang R., Study on Dehydroxylation Process of Kaolinite and its Structural Change. Bulletin of the Chinese Cerami. Society, 1993, 12(6): p.37–41.
Gong Ch., Song X. and Li D., Mechanism Discussion on Calcinated Activate Coal Gangue. Journal of Materials Science. Engineering, 2005, 23(1): p.1–2.
Cheng F., Study on Aluminum Oxide Extracted from Coal Gangue. Chinese Journal of Environmenta. Engineering, 2007, 1(11): p.101–103.
Luo Y., Ma Z. and Wu J., et al. Effect of Different Calcination Temperature on Structure and Extraction Ratio of Alumina from Kaolinite. Chemical Industry and Engineering, 2005, 22(4): p.263–266.
Wang K., Yu Y. and Ou H., 2003. Experimental Study on the Excitation Activity of Low Temperature Calcination of New Grouting Material with Coal Gangue. Chinese Journal of Environmental Engineering, 26 (6): p.4–6.
Si P., Qiao X. and Yu J., Influence of Calcination Atmospheres on Thermal Activation of Kaolinite-Rich Rocks Associated with Coal Measures. Journal of East China Universtiy of Science and Technology (Nature Scienc. Edition, 2011, 05(11): p.571–576.
Li M., and Zhang H. Study on the Effect of Kaolinite on the Combustion Characteristics of Coal. Coa. Convertion, 2004, 27(3): p.68–71.
MacKenzie K., Meinhold R. and Brown I., Theformation of Mullite from Kaolinite under Reaction atmospheres. Journal of the European Cerami. Society, 1996, 16(2): p.115–119.
MacKenzie K., and Meinhold R. The Effect of Reaction Atmospheres on the Early Stage Carbonther-mal Reduction of Kaolinite: An XRD, Si and Al MAS NMR Study. Journal of Materials Science, 1994, 29(21): p.5631–5640.
Cheng F., Study on aluminum oxide extracted from coal gangue. Journal of Environmenta. Engineering, 2007, 1(11): p.99–103.
Acknowledgments
This work is supported by National Natural Science Foundation of China (51074170), Shaanxi Key Technology R & D Program(2016GY-147), and Key Laboratory of Coal Resources Exploration and Comprehensive Utilization, Ministry of Land and Resources Open Research Topic (KF2016-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is supported by National Natural Science Foundation of China (51074170), Shaanxi Key Technology R & D Program(2016GY-147), and Key Laboratory of Coal Resources Exploration and Comprehensive Utilization, Ministry of Land and Resources Open Research Topic (KF2016-3)
Rights and permissions
About this article
Cite this article
Dong, L., Liang, X., Song, Q. et al. Study on Al2O3 extraction from activated coal gangue under different calcination atmospheres. J. Therm. Sci. 26, 570–576 (2017). https://doi.org/10.1007/s11630-017-0975-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11630-017-0975-y