Abstract
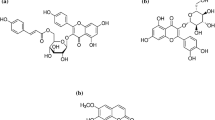
The detection of taxol and related taxanes in Corylus avellana has generated considerable interest, particularly for in vitro cell cultures. Cell suspensions are a sustainable and rational option for obtaining a continuous and reliable source of secondary metabolites in large-scale processes. We therefore focused our study on the main factors that affect the growth of C. avellana cell suspensions as a key approach to improving culture productivity. In this work, calli were successfully induced from C. avellana seeds, leaves, and stems, and the efficiency of different sterilization methods was analyzed. The effects of the basal medium, carbon source, and the type and quantity of plant growth regulators on culture growth were studied. A fractional factorial design allowed us to reduce the number of experiments and analyze all the combinations in one run, thereby reducing time, variability, and costs. Statistical analysis (ANOVA) revealed that 1-naphthaleneacetic acid (NAA) and sucrose are mandatory for the growth of C. avellana cell suspension cultures, with no interactions detected between the parameters analyzed, while growth did not depend on the addition of cytokinins. The secondary metabolism was not inhibited, detecting 1175.45 ng/L of baccatin III and traces of taxol, deacetyltaxol, and cephalomannine. Additionally, prompted by the high growth rate of the C. avellana calli, we assayed a new cold-temperature-based method to maintain a stock of calli using half-strength Murashige and Skoog solid medium, concluding that up to 5 mo at 4°C is optimal to ensure white friable calli upon regrowth at 25°C.



Similar content being viewed by others
References
Asano S, Ohtsubo S, Nakajima M, Kusunoki M, Keneko K, Katayama H, Nawa Y (2002) Production of anthocyanins by habituated cultured cells of Nyoho strawberry (Fragaria ananassa Duch.). Food Sci Technol Res 8:64–69
Bacchetta L, Aramini M, Bernardini C (2008) In vitro propagation of traditional Italian hazelnut cultivars as a tool for the valorization and conservation of local genetic resources. HortSci 43:562–566
Bemani E, Ghanati RA, Jamshidi M (2013) Effect of phenylalanine on taxol production and antioxidant activity of extracts of suspension-cultured hazel (Corylus avellana L) cells. J Nat Med 67:446–451
Bentebibel S, Moyano E, Palazón J, Cusidó RM, Bonfill M, Eibl R, Piñol MT (2005) Effects of immobilization by entrapment in alginate and scale-up on paclitaxel and baccatin III production in cell suspension cultures of Taxus baccata. Biotechnol Bioeng 89:647–655
Bestoso F, Ottaggio L, Armirotti A, Balbi A, Damonte G, Degan P, Mazzei M, Cavalli F, Ledda B, Miele M (2006) In vitro cell cultures obtained from different explants of Corylus avellana produce Taxol and taxanes. BMC Biotechnol 6:45
Binns A, Meins F (1973) Habituation of tobacco pith cells for factors promoting cell division is heritable and potentially reversible. Proc Natl Acad Sci U S A 70:2660–2662
Charlwood BV, Rhodes MJC (1990) Secondary products from plant tissue culture. Clarendon, Oxford
Contessa C, Valentini N, Caviglione M, Botta R (2011) Propagation of Corylus avellana L. by means of semi-hardwood cuttings: rooting and bud retention in four Italian cultivars. Eur J Hortic Sci 76:170–175
Cusidó RM, Palazón J, Bonfill M, Navia-Osorio A, Morales C, Piñol MT (2002) Improved paclitaxel and baccatin III production in suspension cultures of Taxus media. Biotechnol Prog 18:418–423
Cusidó RM, Palazón J, Navia-Osorio A, Mallol A, Bonfill M, Morales C, Piñol MT (1999) Production of Taxol® and baccatin III by a selected Taxus baccata callus line and its derived cell suspension culture. Plant Sci 146:101–107
Diaz-Sala C, Rey M, Rodríguez R (1990) In vitro establishment of a cyclonal chain from nodal segments and apical buds on adult hazel (Corylus avellana L.). Plant Cell Tissue Organ Cult 23:151–157
Dixon RA (2001) Natural products and plant disease resistance. Nature 411:843–847
Eisenhauer EA, Vermorken JB (1998) The taxoids: comparative clinical pharmacology and therapeutic potential. Drugs 55:5–30
Farjaminezhad R, Zare N, Asgahari-Zakaria R, Farjaminezhad M (2013) Establishment and optimization of cell growth in suspension culture of Papaver bracteatum: a biotechnology approach for thebaine production. Turk J Biol 37:689–697
Fornale S, Esposti DD, Navia-Osorio A, Cusido RM, Piñol MT, Bagni N (2002) Taxol transport in Taxus baccata cell suspension cultures. Plant Physiol Biochem 40:81–88
GuiZhi F, YaGuang Z, Bo W, YuTing Y (2009) Effects of light on growth and triterpenes content in callus of Betula platyphylla. J Northeast For Univ 37:1–3
Hartig K, Beck E (2005) Endogenous cytokinin oscillations control cell cycle progression of tobacco BY-2 cells. Plant Biol 7:33–40
Hoffman A, Khan W, Worapong J, Strobel G, Griffin D, Arbogast B, Barofsky B, Boone D, Ning RB, Zheng P, Daley L (1998) Bioprospecting for Taxol in angiosperm plant extracts: using high performance liquid chromatography–thermospray mass spectrometry to detect the anticancer agent and its related metabolites in filbert trees. Spectroscopy 13:22–32
Holmes FA, Walters RS, Theriault RL, Buzdar AU, Fry DK, Hortobagyi GN, Forman AD, Neston LK, Raben MN (1991) Phase II trial taxol, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst 83:1797–1805
Hong S, Xiuxia L, Zhijian X, Dan M, Yinhui L (2010) Callus induction and protoplast isolation from hybrid hazel. J Northeast For Univ 38:23–26
Kajani AA, Moghim S, Mofid MR (2012) Optimization of the basal medium for improving production and secretion of taxanes from suspension cell culture of Taxus baccata L. Daru 20:54
Karwasara VS, Dixit VK (2012) Culture medium optimization for improved puerarin production by cell suspension cultures of Pueraria tuberosa (Roxb. ex Willd.) DC. In Vitro Cell Dev Biol Plant 48:189–199
Karwasara VS, Dixit VK (2013) Culture medium optimization for camptothecin production in cell suspension cultures of Nothapodytes nimmoniana (J. Grah.) Mabberley. Plant Biotechnol Rep 7:357–369
Kevers C, Coumans M, De Greef W, Hofinger M, Gaspar TH (1981) Habituation in sugarbeet callus: auxin content, auxin protectors, peroxidase pattern and inhibitors. Physiol Plant 51:281–286
McGuire WP, Rowinsky EK, Rosenshein HB, Grumbine FC, Ettiger DS, Amstrong DK, Donehower RC (1989) Taxol, a unique antineoplastic agent significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med 111:273–279
Mirjalili HM, Fakhr-Tabatabaei SM, Bonfill M, Alizadeh H, Cusido RM, Ghassempour A, Palazon J (2009) Morphology and withanolide production of Withania coagulans hairy root cultures. Eng Life Sci 9:197–204
Miura GA, Miller CO (1969) Cytokinins from a variant strain of cultured soybean cells. Plant Physiol 44:1035–1039
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Mustafa NR, de Winter W, van Iren F, Verpoorte R (2011) Initiation, growth and cryopreservation of plant cell suspension cultures. Nat Protoc 6:715–742
Nagata T, Nemoto Y, Hasezawa S (1992) Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol 132:1–30
Nagella P, Murthy HN (2010) Establishment of cell suspension cultures of Withania somnifera for the production of withanolide A. Bioresour Technol 101:6735–6739
Nas MN (2004) Inclusion of polyamines in the medium improves shoot elongation in hazelnut (Corylus avellana L.) micropropagation. Turk J Agric For 28:189–194
Nas MN, Read PE (2004) A hypothesis for the development of a defined tissue culture medium of higher plants and micropropagation of hazelnuts. Sci Hortic 101:189–200
Navia-Osorio A, Garden H, Cusidó RM, Palazón J, Alfermann AW, Piñol MT (2002) Production of paclitaxel and baccatin III in a 20-L airlift bioreactor by a cell suspension of Taxus wallichiana. Planta Med 68:336–340
Nishinari N, Syono K (1980) Changes in endogenous cytokinin levels in partially synchronized cultured tobacco cells. Plant Physiol 65:437–441
Ottaggio L, Betoso F, Armirotti A, Balbi A, Damonte G, Mazzei M, Sancandi M, Miele M (2008) Taxanes from shells and leaves of Corylus avellana. J Nat Prod 71:58–60
Pollard JW, Walker JM (1990) Methods in molecular biology, vol 6. Humana Press, Clifton New Jersey
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Redig P, Shaul O, Inze D, Van Montagu M, Van Onckelen H (1996) Levels of endogenous cytokinins, indole-3-acetic acid and abscisic acid during the cell cycle of synchronized tobacco BY-2 cells. FEBS Lett 391:175–180
Reed B, Mentzer J, Trampraset P, Yu X (1998) Internal bacterial contamination of micropropagated hazelnut: identification and antibiotic treatment. Plant Cell Tissue Organ Cult 52:67–70
Rezaei A, Ghanati F, Behmanesh M, Mokhtari-Dizaji M (2011) Ultrasound-potentiated salicylic acid-induced physiological effects and production of taxol in hazelnut (Corylus avellana L.) cell culture. Ultrasound Med Biol 37:1938–1947
Rezaei A, Ghanati F, Behmanesh M, Safari M, Sharafi Y (2013) Synergistic accumulative effect of salicylic acid and dibutyl phthalate on paclitaxel production in Corylus avellana cell culture. J Stress Physiol Biochem 9:157–168
Richheimer SL, Tinnermeier DM, Timmons DW (1992) High-performance liquid chromatographic assay of taxol. Anal Chem 64:2323–2326
Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14:S185–S205
Safari M, Ghanati F, Hajnoruzi A, Rezaei A, Abdolmaleki P, Mokhtari-Dizaji M (2012) Maintenance of membrane integrity and increase of taxanes production in hazel (Corylus avellana L.) cells induced by low-intensity ultrasounds. Biotechnol Lett 34:1137–1141
Sanchez-Olate M, Rios D, Rodriguez R, Materan ME, Pereira G (2004) Duration of the reinvigorating effect of severe pruning of mature European hazelnut plants (Corylus avellana L.) cv. Negretta with in vitro cultivation. Agric Technol 64:338–346
Saville M, Lietzau J, Pluda J, Feuerstein I, Odom J, Wilson W, Humphrey R, Feigal E, Steinberg S, Broder S (1995) Treatment of HIV-associated Kaposi’s sarcoma with paclitaxel. Lancet 346:26–28
Singh M, Chaturvedi R (2012) Evaluation of nutrient uptake and physical parameters on cell biomass growth and production of spilanthol in suspension cultures of Spilanthes acmella Murr. Bioprocess Biosyst Eng 35:943–951
Sivanandhan G, Kapil Dev G, Jeyaraj M, Rajesh M, Muthuselvam M, Selvaraj N, Manickavasagam M, Ganapathi A (2013) A promising approach on biomass accumulation and withanolides production in cell suspension culture of Withania somnifera (L.) Dunal. Protoplasma 250:885–898
Suehara K, Kameoka T, Hashimoto A (2012) Sugar uptake analysis of suspension Arabidopsis, tobacco, and rice cells in various media using an FT-IR/ATR method. Bioprocess Biosyst Eng 35:1259–1268
Sung LS, Huang SY (2000) Medium optimization of transformed root cultures of Stizolobium hassjoo producing L-DOPA with response surface methodology. Biotechnol Prog 16:1135–1140
Vasilev N, Grömping U, Lipperts A, Raven N, Fischer R, Schillberg S (2013) Optimization of BY-2 cell suspension culture medium for the production of a human antibody using a combination of fractional factorial designs and the response surface method. Plant Biotechnol J 11:867–874
Verpoorte R (2000) Secondary metabolisms. In: Verpoorte R, Alfermann AW (eds) Metabolic engineering of plant secondary metabolism. Kluwer Academic Publishers, London, pp 1–29
Wyndaele R, Christiansen J, Horseele R, Rüdelsheim P, Van Onckelen H (1988) Functional correlation between endogenous phytohormone levels and hormone autotrophy of transformed and habituated soybean cell lines. Plant Cell Physiol 29:1095–1101
Yu X, Reed BM (1995) A micropropagation system for hazelnuts (Corylus species). HortSci 30:120–123
Acknowledgments
Work in the Plant Physiology Laboratory (University of Barcelona) was financially supported by the Spanish MEC (BIO2011-29856-C02-1) and the Generalitat de Catalunya (2014SGR215). A. Gallego held a grant from the Universitat Pompeu Fabra.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Forster
Rights and permissions
About this article
Cite this article
Gallego, A., Bonfill, M., Cusido, R.M. et al. Assessing factors that affect the growth of Corylus avellana cell suspension cultures: a statistical approach. In Vitro Cell.Dev.Biol.-Plant 51, 530–538 (2015). https://doi.org/10.1007/s11627-015-9693-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9693-x