Summary
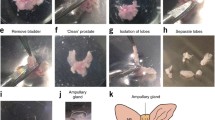
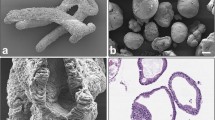
A system has been developed for the culture of cells that provides conditions favoring the formation of tissues comparable to conditions existing in nature. The culture chamber is a lens-shaped pouch composed of two thin-walled, reinforced, waffled collagen membranes facing each other. The chamber is immersed in immersed in medium in a closed transparent container and incubated on a rocker. On histologic study, after days to weeks in culture, human mammary cancer cell lines BT-20, MCF-7, MDA-231, MDA-468, and T47D grow in the chamber as distinctive structured epithelial tissue. Dog kidney cell line MDCK grows as a papillary adenocarcinoma and rat bladder cancer line NBT-II as an epidermoid carcinoma; cells from clinical effusion tumors produce distinct tissue. Changes in histologic phenotype may be driven by molecular changes at the level of the genome. Resulting alteration of the biochemical functions essential for the integrity of specific durable tissue organization should alter or reset the pattern of tissue organization and of biological behavior, including malignancy and response to cytotoxic chemicals. Lenticular pouch culture promises to be an effective tool for exploring the molecular changes associated with histogenesis and malignancy.
Similar content being viewed by others
References
Leighton, J. A sponge matrix method for tissue culture. Formation of organized aggregates of cells in vitro. J.N.C.I. 12:545–561; 1951.
Leighton, J. Studies on human cancer using sponge matrix tissue culture I. The growth patterns of a malignant melanoma, adenocarcinoma of the parotid gland, papillary adenocarcinoma of the thyroid gland, adenocarcinoma of the pancreas and epidermoid carcinoma of the uterine cervix (Gey’s HeLa Strain). Texas Rep. Biol. Med. 12:847–864; 1954.
Leighton, J. The growth patterns of some transplantable animal tumors in sponge matrix tissue culture. J. Natl. Cancer Inst. 15:275–293; 1954.
Leighton, J. Economic maintenance of continuous lines of human cells with bead-in-tube cultures. Lab. Invest. 7:513–514; 1958.
Leighton, J. Neoplastic blockade, a new concept in the destruction of normal tissue by cancer. In: Katsuta, H., ed. Cancer cells in culture. Tokyo, Japan: University of Tokyo Press; 1968:143–156.
Leighton, J. Radial histophysiological gradient culture chamber: rationale and preparation. In Vitro Cell. Dev. Biol. 27A:786–790; 1991.
Leighton, J. Structural biology of epithelial tissue in histophysiologic gradient culture. In Vitro Cell. Dev. Biol. 28A:482–492; 1992.
Leighton, J.; Justh, G.; Esper, M., et al. Collagen-coated cellulose sponge: three-dimensional matrix for tissue culture of Walker tumor 256. Science 155:1259–1261; 1967.
Leighton, J.; Mark, R.; Justh, G. Patterns of three-dimensional growth in vitro in collagen-coated cellulose sponge: carcinomas and embryonic tissues. Cancer Res. 28:286–296; 1968.
Leighton, J.; Matulaitis, R. Histotypic organization of carcinoma in radial gradient culture chamber. Clin. Biotechnol. 2:17–21; 1990.
Leighton, J.; Tchao, R.; Nichols, J. Radial gradient culture on the inner surface of collagen tubes: organoid growth of normal rat bladder and rat bladder cancer cell line NBT-II. In Vitro Cell. Dev. Biol. 21:713–715; 1985.
Leighton, J.; Tchao, R.; Tencer, K. L. Organoid structure of normal rat bladder in unilaminar and bilaminar histophysiologic gradient culture: methods and observations. In Vitro 20:183–197; 1984.
Roberts, D. D.; Leighton, J.; Abaza, N. A., et al. Heterotopic urinary bladders in rats produced by an isograft inoculum of bladder fragments and air. Cancer Res. 34:2773–2778; 1974.
Wolff, E. T.; Wolff, E. M. Current research with organ cultures of human tumors. In: Fogh, J., ed. Human tumor cells in vitro. New York: Plenum Press; 1975:207–240.
Author information
Authors and Affiliations
Additional information
This paper is dedicated to the memory of Clyde J. Dawe, who died suddenly on Cape Cod, Massachusetts, in a glider accident on July 5, 1996. We were friends for about 40 years. Clyde was one of the finest scholars in cancer biology I have ever known. I learned many things from him and miss him.
Rights and permissions
About this article
Cite this article
Leighton, J. Human mammary cancer cell lines and other epithelial cells cultured as organoid tissue in lenticular pouches of reinforced collagen membranes. In Vitro Cell.Dev.Biol.-Animal 33, 783–790 (1997). https://doi.org/10.1007/s11626-997-0157-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11626-997-0157-4