Abstract
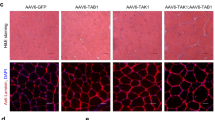
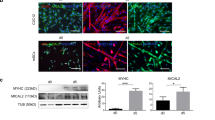
Skeletal muscle tissue increases or decreases its volume by synthesizing or degrading myofibrillar proteins. The ubiquitin–proteasome system plays a pivotal role during muscle atrophy, where muscle ring finger proteins (Murf) function as E3 ubiquitin ligases responsible for identifying and targeting substrates for degradation. Our previous study demonstrated that overexpression of Ozz, an E3 specific to embryonic myosin heavy chain (Myh3), precisely reduced the Myh3 replacement rate in the thick filaments of myotubes (E. Ichimura et al., Physiol Rep. 9:e15003, 2021). These findings strongly suggest that E3 plays a critical role in regulating myosin replacement. Here, we hypothesized that the Murf isoforms, which recognize Myhs as substrates, reduced the myosin replacement rates through the enhanced Myh degradation by Murfs. First, fluorescence recovery after a photobleaching experiment was conducted to assess whether Murf isoforms affected the GFP-Myh3 replacement. In contrast to Murf2 or Murf3 overexpression, Murf1 overexpression selectively facilitated the GFP-Myh3 myosin replacement. Next, to examine the effects of Murf1 overexpression on the replacement of myosin isoforms, Cherry-Murf1 was coexpressed with GFP-Myh1, GFP-Myh4, or GFP-Myh7 in myotubes. Intriguingly, Murf1 overexpression enhanced the myosin replacement of GFP-Myh4 but did not affect those of GFP-Myh1 or GFP-Myh7. Surprisingly, overexpression of Murf1 did not enhance the ubiquitination of proteins. These results indicate that Murf1 selectively regulated myosin replacement in a Myh isoform–dependent fashion, independent of enhanced ubiquitination. This suggests that Murf1 may have a role beyond functioning as a ubiquitin ligase E3 in thick filament myosin replacement.






Similar content being viewed by others
References
Aweida D, Cohen S (2021) Breakdown of filamentous myofibrils by the UPS-step by step. Biomolecules 11:110
Baehr LM, Furlow JD, Bodine SC (2011) Muscle sparing in muscle RING finger 1 null mice: response to synthetic glucocorticoids. J Physiol 589:4759–4776
Baehr LM, Hughes DC, Lynch SA, Van Haver D, Maia TM, Marshall AG, Radoshevich L, Impens F, Waddell DS, Bodine SC (2021) Identification of the MuRF1 skeletal muscle ubiquitylome through quantitative proteomics. Function (Oxf) 2:zqab029
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Cecile P, Julien A, Andrea A, Agnes C, Cecile CG, Clara T, Christiane D, Lydie C, Daniel B, Marco S, Didier A, Daniel T (2018) UBE2E1 is preferentially expressed in the cytoplasm of slow-twitch fibers and protects skeletal muscles from exacerbated atrophy upon dexamethasone treatment. Cells 7:214
Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S (2001) Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol 306:717–726
Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ (2007) The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab 6:376–385
Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL (2009) During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol 185:1083–1095
Craig R, Offer G (1976) Axial arrangement of crossbridges in thick filaments of vertebrate skeletal muscle. J Mol Biol 102:325–332
Fielitz J, Kim MS, Shelton JM, Latif S, Spencer JA, Glass DJ, Richardson JA, Bassel-Duby R, Olson EN (2007) Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J Clin Invest 117:2486–2495
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984
Henderson CA, Gomez CG, Novak SM, Mi-Mi L, Gregorio CC (2017) Overview of the muscle cytoskeleton. Compr Physiol 7:891–944
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479
Hirner S, Krohne C, Schuster A, Hoffmann S, Witt S, Erber R, Sticht C, Gasch A, Labeit S, Labeit D (2008) MuRF1-dependent regulation of systemic carbohydrate metabolism as revealed from transgenic mouse studies. J Mol Biol 379:666–677
Hyatt HW, Powers SK (2020) The role of calpains in skeletal muscle remodeling with exercise and inactivity-induced atrophy. Int J Sports Med 41:994–1008
Ichimura E, Ojima K, Muroya S, Suzuki T, Kobayashi K, Nishimura T (2021) The ubiquitin ligase Ozz decreases the replacement rate of embryonic myosin in myofibrils. Physiol Rep 9:e15003
Koyama S, Hata S, Witt CC, Ono Y, Lerche S, Ojima K, Chiba T, Doi N, Kitamura F, Tanaka K, Abe K, Witt SH, Rybin V, Gasch A, Franz T, Labeit S, Sorimachi H (2008) Muscle RING-finger protein-1 (MuRF1) as a connector of muscle energy metabolism and protein synthesis. J Mol Biol 376:1224–1236
Labeit S, Kolmerer B (1995) Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science 270:293–296
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18:39–51
Luther PK, Munro PM, Squire JM (1981) Three-dimensional structure of the vertebrate muscle A-band. III. M-region structure and myosin filament symmetry. J Mol Biol 151:703–730
Maruyama K (1976) Connectin, an elastic protein from myofibrils. J Biochem 80:405–407
McElhinny AS, Kakinuma K, Sorimachi H, Labeit S, Gregorio CC (2002) Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1. J Cell Biol 157:125–136
McElhinny AS, Perry CN, Witt CC, Labeit S, Gregorio CC (2004) Muscle-specific RING finger-2 (MURF-2) is important for microtubule, intermediate filament and sarcomeric M-line maintenance in striated muscle development. J Cell Sci 117:3175–3188
Mizushima N (2011) Autophagy in protein and organelle turnover. Cold Spring Harb Symp Quant Biol 76:397–402
Ojima K, Ichimura E, Suzuki T, Oe M, Muroya S, Nishimura T (2018) HSP90 modulates the myosin replacement rate in myofibrils. Am J Physiol Cell Physiol 315:C104–C114
Ojima K, Ichimura E, Yasukawa Y, Wakamatsu J, Nishimura T (2015) Dynamics of myosin replacement in skeletal muscle cells. Am J Physiol Cell Physiol 309:C669-679
Ojima K, Kigaki M, Ichimura E, Suzuki T, Kobayashi K, Muroya S, Nishimura T (2022) Endogenous slow and fast myosin dynamics in myofibers isolated from mice expressing GFP-Myh7 and Kusabira Orange-Myh1. Am J Physiol Cell Physiol 323:C520–C535
Ojima K, Lin ZX, Bang M, Holtzer S, Matsuda R, Labeit S, Sweeney HL, Holtzer H (2000) Distinct families of Z-line targeting modules in the COOH-terminal region of nebulin. J Cell Biol 150:553–566
Perera S, Mankoo B, Gautel M (2012) Developmental regulation of MURF E3 ubiquitin ligases in skeletal muscle. J Muscle Res Cell Motil 33:107–122
Peris-Moreno D, Malige M, Claustre A, Armani A, Coudy-Gandilhon C, Deval C, Bechet D, Fafournoux P, Sandri M, Combaret L, Taillandier D, Polge C (2021) UBE2L3, a partner of MuRF1/TRIM63, is involved in the degradation of myofibrillar actin and myosin. Cells 10:1974
Peris-Moreno D, Taillandier D, Polge C (2020) MuRF1/TRIM63, master regulator of muscle mass. Int J Mol Sci 21:6663
Polge C, Heng AE, Jarzaguet M, Ventadour S, Claustre A, Combaret L, Bechet D, Matondo M, Uttenweiler-Joseph S, Monsarrat B, Attaix D, Taillandier D (2011) Muscle actin is polyubiquitinylated in vitro and in vivo and targeted for breakdown by the E3 ligase MuRF1. FASEB J 25:3790–3802
Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3:1009–1013
Spencer JA, Eliazer S, Ilaria RL Jr, Richardson JA, Olson EN (2000) Regulation of microtubule dynamics and myogenic differentiation by MURF, a striated muscle RING-finger protein. J Cell Biol 150:771–784
Sweeney HL, Hammers DW (2018) Muscle contraction. Cold Spring Harb Perspect Biol 10:a023200
Tawa NE Jr, Odessey R, Goldberg AL (1997) Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J Clin Invest 100:197–203
Wang K, McClure J, Tu A (1979) Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci U S A 76:3698–3702
Witt CC, Witt SH, Lerche S, Labeit D, Back W, Labeit S (2008) Cooperative control of striated muscle mass and metabolism by MuRF1 and MuRF2. EMBO J 27:350–360
Witt SH, Granzier H, Witt CC, Labeit S (2005) MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol 350:713–722
Acknowledgements
We sincerely appreciate the valuable technical support provided by Miho Ichimura (National Institute of Livestock and Grass Sciences, NARO).
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant No. 19H03100 to KO and TN and Grant No. 23H03277 to KO.
Author information
Authors and Affiliations
Contributions
KO and TN conceived and designed the research. EU and KO conducted the experiments; EU, KO, TS, KK, SM, and TN analyzed the data; EU, KO, and TN interpreted the results of the experiments. EU and KO prepared the figures, and EU and KO drafted the manuscript. EU, KO, TS, KK, SM, and TN edited and revised the manuscript. EU, KO, TS, KK, SM, and TN approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uenaka, E., Ojima, K., Suzuki, T. et al. Murf1 alters myosin replacement rates in cultured myotubes in a myosin isoform–dependent manner. In Vitro Cell.Dev.Biol.-Animal (2024). https://doi.org/10.1007/s11626-024-00916-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11626-024-00916-0