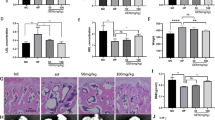
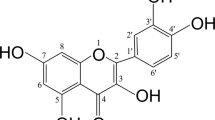
Abstract
Osteoporosis is a metabolic condition distinguished by the degradation of bone microstructure and mechanical characteristics. Traditional Chinese medicine (TCM) has been employed in China for the treatment of various illnesses. Naringin, an ingredient found in Drynariae TCM, is known to have a significant impact on bone metabolism. For this research, we studied the precise potential effect of Drynaria Naringin on protecting against bone loss caused by stress deficiency. In this study, a tail-suspension (TS) test was performed to establish a mouse model with hind leg bone loss. Some mice received subcutaneous injections of Drynaria Naringin for 30 d. Trabecular bone microarchitecture was evaluated using micro-computed tomography analysis and bone histological analysis. Bone formation and resorption markers were quantified in blood samples from mice or in the supernatant of MC3T3-E1 cells by ELISA analysis, Western blotting, and PCR. Immunofluorescence was utilized to visualize the location of β-catenin. Additionally, siRNA was employed to knockdown-specific genes in the cells. Our findings highlight the efficacy of Drynaria Naringin in protecting against the deterioration of bone loss and promoting bone formation and Rspo1 expression in a mouse model following the TS test. Specifically, in vitro experiments also indicated that Drynaria Naringin may promote osteogenesis through the Wnt/β-catenin signalling pathway. Moreover, our results suggest that Drynaria Naringin upregulates the expression of Rspo1/Lgr4, leading to the promotion of osteogenesis via the Wnt/β-catenin signalling pathway. Therefore, Drynaria Naringin holds potential as a therapeutic medication for osteoporosis. Drynaria Naringin alleviates bone loss deterioration caused by mechanical stress deficiency through the Rspo1/Lgr4-mediated Wnt/β-catenin signalling pathway.






Similar content being viewed by others
Data availability
The data underlying this article are available from the corresponding author, Ze-Zhu Zhou.
References
Albus U (2012) Guide for the care and use of laboratory animals. Lab Anim 46:267–268
Alexandre C, Vico L (2011) Pathophysiology of bone loss in disuse osteoporosis. Joint Bone Spine 78:572–576
Ang E, Yang X, Chen H et al (2011) Naringin abrogates osteoclastogenesis and bone resorption via the inhibition of RANKL-induced NF-κB and ERK activation. FEBS Lett 585:2755–2762
Bilezikian JP (2016) Osteonecrosis of the jaw-do bisphosphonates pose a risk? N Engl J Med 355:2278–2281
Chen LL, Lei LH, Ding PH et al (2011) Osteogenic effect of Drynariae rhizoma extracts and Naringin on MC3T3-E1 cells and an induced rat alveolar bone resorption model. Arch Oral Biol 56:1655–1662
Chen R, Qi Q, Wang M et al (2016) Therapeutic potential of Naringin: an overview. Pharm Biol 54:3203–3210
de Lau W, Barker N, Low TY et al (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476:293–297
Duncan RL, Turner CH (1995) Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 57:344–358
Gallacher SJ, Dixon T (2010) Impact of treatments for postmenopausal osteoporosis (bisphosphonates, parathyroid hormone, strontium ranelate, and denosumab) on bone quality: A systematic review. Calcif Tissue Int 87:469–484
Gutzwiller JP, Richterich JP, Stanga Z et al (2018) Osteoporosis, diabetes, and hypertension are major risk factors for mortality in older adults: an intermediate report on a prospective survey of 1467 community-dwelling elderly healthy pensioners in Switzerland. BMC Geriatr 18:115
Hernlund E, Svedbom A, Ivergard M et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 8:136
Hu H, Chen Y, Zou Z et al (2020) Panax notoginseng saponins prevent bone loss by promoting angiogenesis in an osteoporotic mouse model. Biomed Res Int 2020:1–8
Huang S, Lin L, Wang S et al (2023) Total flavonoids of rhizoma Drynariae mitigates aflatoxin B1-induced liver toxicity in chickens via microbiota-gut-liver axis interaction mechanisms. Antioxidants (basel) 12:819
Huang Y, Yang C, Chiou Y (2011) Citrus flavanone naringenin enhances melanogenesis through the activation of Wnt/β-catenin signalling in mouse melanoma cells. Phytomedicine 18:1244–1249
Kohrt WM, Bloomfield SA, Little KD et al (2004) Physical activity and bone health. Med Sci Sports Exerc 36:1985–1996
LeBlanc AD, Spector ER, Evans HJ et al (2007) Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact 7:33–47
Lewiecki ME (2009) Current and emerging pharmacologic therapies for the management of postmenopausal osteoporosis. J Womens Health 18:1615–1626
Li CY, Price C, Delisser K et al (2005) Long-term disuse osteoporosis seems less sensitive to bisphosphonate treatment than other osteoporosis. J Bone Miner Res 20:117–124
Li F, Sun X, Ma J et al (2014) Naringin prevents ovariectomy-induced osteoporosis and promotes osteoclasts apoptosis through the mitochondria-mediated apoptosis pathway. Biochem Biophys Res Commun 452:629–635
Li N, Jiang Y, Wooley PH et al (2013) Naringin promotes osteoblast differentiation and effectively reverses ovariectomy-associated osteoporosis. J Orthop Sci 18:478–485
Lin FX, Du SX, Liu DZ et al (2016) Naringin promotes osteogenic differentiation of bone marrow stromal cells by up-regulating Foxc2 expression via the IHH signaling pathway. Am J Transl Res 8:5098–5107
Lin J, Zhu J, Wang Y et al (2017) Chinese single herbs and active ingredients for postmenopausal osteoporosis: from preclinical evidence to action mechanism. Biosci Trends 11:496–506
Liu M, Li Y, Yang S (2017) Effects of Naringin on the proliferation and osteogenic differentiation of human amniotic fluid-derived stem cells. J Tissue Eng Regen Med 11:276–284
Luo J, Zhou W, Zhou X et al (2009) Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development 136:2747–2756
Lv J, Sun X, Ma J et al (2015) Involvement of periostin-sclerostin-Wnt/β-catenin signalling pathway in the prevention of neurectomy-induced bone loss by Naringin. Biochem Biophys Res Commun 468:587–593
Ma X, Lv J, Sun X et al (2016) Naringin ameliorates bone loss induced by sciatic neurectomy and increases Semaphorin 3A expression in denervated bone. Sci Rep 6:24562
McClung M, Harris ST, Miller PD et al (2013) Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 126:13–20
Moreira LDF, de Oliveira ML, Lirani-Galv AP et al (2014) Physical exercise and osteoporosis: effects of different types of exercises on bone and physical function of postmenopausal women. Arq Bras Endocrinol Metabol 58:514–522
Rivoira M, Rodriguez V, Picotto G et al (2018) Naringin prevents bone loss in a rat model of type 1 Diabetes mellitus. Arch Biochem Biophys 637:56–63
Robling AG, Niziolek PJ, Baldridge LA et al (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283:5866–5875
Shi GX, Mao WW, Zheng XF et al (2016) The role of R-spondins and their receptors in bone metabolism. Prog Biophys Mol Biol 122:93–100
Siu WS, Ko CH, Hung LK et al (2013) Effect of anti-osteoporotic agents on the prevention of bone loss in unloaded bone. Mol Med Rep 8:1188–1194
Song N, Zhao Z, Ma X et al (2017) Naringin promotes fracture healing through stimulation of angiogenesis by regulating the VEGF/VEGFR-2 signalling pathway in osteoporotic rats. Chem Biol Interact 261:11–17
Song S, Gao Z, Lei X et al (2017) Total flavonoids of Drynariae rhizoma prevent bone loss induced by hindlimb unloading in rats. Molecules 22:1033.
Song SH, Zhai YK, Li CQ et al (2016) Effects of total flavonoids from Drynariae Rhizoma prevent bone loss in vivo and in vitro. Bone Rep 5:262–273
Tang JL, Liu BY, Ma KW (2008) Traditional Chinese Medicine. The Lancet 372:1938–1940
Turner CH (1998) Three rules for bone adaptation to mechanical stimuli. Bone 23:399–407
Unnanuntana A, Gladnick BP, Donnelly E et al (2010) The assessment of fracture risk. J Bone Joint Surg Am 92:743–753
Vestergaard P, Rejnmark L, Mosekilde L (2007) Increased mortality in patients with a hip fracture-effect of pre-morbid conditions and post-fracture complications. Osteoporos 18:1583–1593
Wang D, Ma W, Wang F et al (2015) Stimulation of Wnt/β-Catenin signalling to improve bone development by Naringin via interacting with AMPK and Akt. Cell Physiol Biochem 36:1563–1576
Wang H, Li C, Li J et al (2017) Naringin enhances osteogenic differentiation through the activation of ERK signalling in human bone marrow mesenchymal stem cells. Iran J Basic Med Sci 20:408–414
Warden SJ, MantilaRoosa SM, Kersh ME et al (2014) Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci USA 111:5337–5342
Wei M, Yang Z, Li P et al (2007) Anti-osteoporosis activity of Naringin in the retinoic acid-induced osteoporosis model. Am J Chin Med 35:663–667
Willson T, Nelson SD, Newbold J et al (2015) The clinical epidemiology of male osteoporosis: A review of the recent literature. Clin Epidemiol 7:65–76
Wu L, Ling Z, Feng X et al (2017) Herb Medicines against osteoporosis: Active compounds & relevant biological mechanisms. Curr Top Med Chem 17:1670–1691
Xu T, Wang L, Tao Y et al (2016) The function of Naringin in inducing secretion of osteoprotegerin and inhibiting formation of osteoclasts. Evid Based Complement Alternat Med 2016:8981650
Yu GY, Zheng GZ, Chang B et al (2016) Naringin stimulates osteogenic differentiation of rat bone marrow stromal cells via activation of the Notch signaling pathway. Stem Cells Int 2016:7130653
Zhang ND, Han T, Huang BK et al (2016) Traditional Chinese medicine formulas for the treatment of osteoporosis: implication for antiosteoporotic drug discovery. J Ethnopharmacol 189:61–80
Zhu C, Zheng XF, Yang YH et al (2016) LGR4 acts as a key receptor for R-spondin 2 to promote osteogenesis through Wnt signalling pathway. Cell Signal 28:989–1000
Funding
This work was supported by the Nature Science Foundation of Jiading district of Shanghai in China (JDKW-2020–0030).
Author information
Authors and Affiliations
Contributions
Ze-Zhu Zhou conceived and designed the study. Gui-Xun Shi and Wei-Dong Sun performed the experiments and wrote the paper. Zeng-Huan Chen analysed the data. Chuan-Jun Yang organized the figures. Wang-Lin Luo and Dan-Feng Wang did the manuscript revision. Ze-Zhu Zhou supervised the whole study. All authors have read and approved the manuscript.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, GX., Sun, WD., Chen, ZH. et al. Drynaria Naringin alleviated mechanical stress deficiency-caused bone loss deterioration via Rspo1/Lgr4-mediated Wnt/β-catenin signalling pathway. In Vitro Cell.Dev.Biol.-Animal 59, 706–716 (2023). https://doi.org/10.1007/s11626-023-00815-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-023-00815-w