Abstract
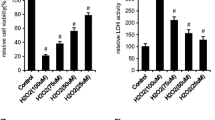
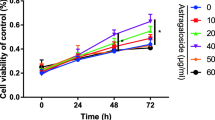
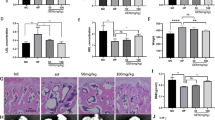
This work aims to study the function of curculigoside in osteoporosis and explore whether DNMT1 is closely involved in osteoblast activity. After OB-6 osteoblasts were treated with hydrogen peroxide (H2O2), a curculigoside treatment group was set up and a series of biological tests including MTT, flow cytometry, western blotting, ROS fluorescence intensity, mitochondrial membrane potential, and ELISA experiments were performed to verify the effect of curculigoside on the activity of osteoblasts. Then, alkaline phosphatase (ALP) activity, alizarin red staining, PCR, and western blotting assays were performed to detect the effects of curculigoside on osteoblast function. By constructing DNMT1 knockdown and overexpression OB-6 cell lines, the effect of DNMT1 on osteoblast function was verified. In addition, the expression level of Nrf2 in each group was detected to speculate the mechanism of DNMT1 in osteoporosis. The cell activity and level of bcl-2 and SOD were significantly increased; the cell apoptosis, ROS fluorescence intensity, mitochondrial membrane potential, MDA and level of caspase-3, Bax, and CAT was reduced in curculigoside treatment group compared with H2O2-induced OB-6 osteoblasts. Meanwhile, the ALP activity, number and area of bone mineralized nodules, and gene and protein expression of OSX and OPG were significantly elevated in curculigoside group. Moreover, DNMT1 knockdown had a similar promotion effect on osteoblast function as curculigoside, and DNMT1 overexpression could reverse the promotion effect of curculigoside on osteoblast function. Further mechanistic studies speculated that DNMT1 might play a role in osteoporosis by affecting Nrf2 methylation. Curculigoside enhances osteoblast activity through DNMT1 controls of Nrf2 methylation.






Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article.
References
Agidigbi TS, Kim C (2019) Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int J Mol Sci 20:3576
Alexiou KI, Roushias A, Varitimidis SE, Malizos KN (2018) Quality of life and psychological consequences in elderly patients after a hip fracture: a review. Clin Interv Aging 13:143–150
Chen W, Wu P, Yu F, Luo G, Qing L, Tang J (2022) HIF-1α regulates bone homeostasis and angiogenesis, participating in the occurrence of bone metabolic diseases. Cells 11:3552
Chen X, Chen J, Xu D, Zhao S, Song H, Peng Y (2017) Effects of osteoglycin (OGN) on treating senile osteoporosis by regulating MSCs. BMC Musculoskelet Disord 18:423
Chen X, Zhu X, Wei A, Chen F, Gao Q, Lu K, Jiang Q, Cao W (2021) Nrf2 epigenetic derepression induced by running exercise protects against osteoporosis. Bone Res 9:15
Cymet TC, Wood B, Orbach N (2000) Osteoporosis. J Am Osteopath Assoc 100:S9-15
Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B (2019) Oxidative stress in chronic kidney disease. Pediatr Nephrol 34:975–991
Fang H, Deng Z, Liu J, Chen S, Deng Z, Li W (2022) The mechanism of bone remodeling after bone aging. Clin Interv Aging 17:405–415
Fischer V, Haffner-Luntzer M (2022) Interaction between bone and immune cells: implications for postmenopausal osteoporosis. Semin Cell Dev Biol 123:14–21
Guo S, Chen C, Ji F, Mao L, Xie Y (2017) PP2A catalytic subunit silence by microRNA-429 activates AMPK and protects osteoblastic cells from dexamethasone. Biochem Biophys Res Commun 487:660–665
Gosset A, Pouillès JM, Trémollieres F (2021) Menopausal hormone therapy for the management of osteoporosis. Best Pract Res Clin Endocrinol Metab 35:101551
Han J, Wan M, Ma Z, Hu C, Yi H (2020) Prediction of targets of curculigoside A in osteoporosis and rheumatoid arthritis using network pharmacology and experimental verification. Drug Des Devel Ther 14:5235–5250
Kimball JS, Johnson JP, Carlson DA (2021) Oxidative stress and osteoporosis. J Bone Joint Surg Am 103:1451–1461
Lai EC, Lin TC, Lange JL, Chen L, Wong ICK, Sing CW, Cheung CL, Shao SC, Yang YK (2022) Effectiveness of denosumab for fracture prevention in real-world postmenopausal women with osteoporosis: a retrospective cohort study. Osteoporos Int 33:1155–1164
Lephart ED, Naftolin F (2021) Menopause and the skin: old favorites and new innovations in cosmeceuticals for estrogen-deficient skin. Dermatol Ther (heidelb) 11:53–69
Lian WS, Wu RW, Chen YS, Ko JY, Wang SY, Jahr H, Wang FS (2021) MicroRNA-29a mitigates osteoblast senescence and counteracts bone loss through oxidation resistance-1 control of FoxO3 methylation. Antioxidants (Basel) 10:1248
Liu M, Liu S, Zhang Q, Fang Y, Yu Y, Zhu L, Liu Y, Gong W, Zhao L, Qin L, Zhang Q (2021) Curculigoside attenuates oxidative stress and osteoclastogenesis via modulating Nrf2/NF-κB signaling pathway in RAW264.7 cells. J Ethnopharmacol 275:114129
McDonald MM, Kim AS, Mulholland BS, Rauner M (2021) New insights into osteoclast biology. JBMR plus 5:e10539
Mohan KN (2022) DNMT1: catalytic and non-catalytic roles in different biological processes. Epigenomics 14:629–643
Svedružić ŽM (2011) Dnmt1 structure and function. Prog Mol Biol Transl Sci 101:221–254
Torres ML, Wanionok NE, McCarthy AD, Morel GR, Fernández JM (2021) Systemic oxidative stress in old rats is associated with both osteoporosis and cognitive impairment. Exp Gerontol 156:111596
Valls J, Richard T, Larronde F, Leblais V, Muller B, Delaunay JC, Monti JP, Ramawat KG, Mérillon JM (2006) Two new benzylbenzoate glucosides from Curculigo orchioides. Fitoterapia 77:416–419
Wang YK, Hong YJ, Wei M, Wu Y, Huang ZQ, Chen RZ, Chen HZ (2010) Curculigoside attenuates human umbilical vein endothelial cell injury induced by H2O2. J Ethnopharmacol 132:233–239
Zhang Q, Zhao L, Shen Y, He Y, Cheng G, Yin M, Zhang Q, Qin L (2019) Curculigoside protects against excess-iron-induced bone loss by attenuating Akt-FoxO1-dependent oxidative damage to mice and osteoblastic MC3T3-E1 cells. Oxid Med Cell Longev 2019:9281481
Zhao L, Liu S, Wang Y, Zhang Q, Zhao W, Wang Z, Yin M (2015) Effects of curculigoside on memory impairment and bone loss via anti-oxidative character in APP/PS1 mutated transgenic mice. PLoS ONE 10:e0133289
Zuo AX, Shen Y, Jiang ZY, Zhang XM, Zhou J, Lü J, Chen JJ (2010) Three new phenolic glycosides from Curculigo orchioides G. Fitoterapia 81:910–913
Acknowledgements
Not applicable.
Funding
This work is supported by Postdoctoral Program of Shandong University of Traditional Chinese Medicine and Hospital-level Research Project of Rizhao Hospital of Traditional Chinese Medicine (No. RZZY2023JC01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Cui, K., Guo, J. et al. Curculigoside attenuates osteoporosis through regulating DNMT1 mediated osteoblast activity. In Vitro Cell.Dev.Biol.-Animal 59, 649–657 (2023). https://doi.org/10.1007/s11626-023-00813-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-023-00813-y