Abstract
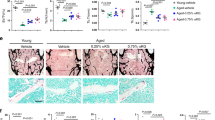
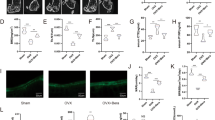
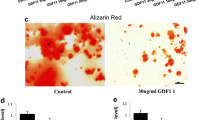
The role of kynurenic acid (KynA) in neurological and mental diseases has been widely studied. Emerging studies disclosed that KynA has a protective effect on tissues including heart, kidney, and retina. However, the role of KynA in osteoporosis has not been reported so far. To elucidate the role of KynA in age-related osteoporosis, both control and osteoporosis mice were administrated KynA for three consecutive months, and micro-computed tomography (μCT) analysis was then performed. In addition, primary bone marrow mesenchymal stem cells (BMSCs) were isolated for osteogenic differentiation induction and treated with KynA in vitro. Our data suggested that KynA administration rescued age-related bone loss in vivo, and KynA treatment promotes BMSC osteogenic differentiation in vitro. Moreover, KynA activated the Wnt/β-catenin signaling during BMSC osteogenic differentiation. Wnt inhibitor MSAB inhibited KynA-induced osteogenic differentiation. Further data demonstrated that KynA exerted its effect on BMSC osteogenic differentiation and Wnt/β-catenin signaling activation via G protein-coupled receptor 35 (GPR35). In conclusion, the protective effect of KynA on age-related osteoporosis was disclosed. Additionally, the promoting effect of KynA on osteoblastic differentiation via Wnt/β-catenin signaling was verified and the effect dependent on GPR35. These data suggest that KynA administration potentially contributes to the treatment of age-related osteoporosis.





Similar content being viewed by others
Data availability
Data will be made available upon reasonable request.
References
Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL (2009) Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem 284:27438–27448
Anaya JM, Bollag WB, Hamrick MW, Isales CM (2020) The role of tryptophan metabolites in musculoskeletal stem cell aging. Int J Mol Sci 21:6670
Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L, Yang H, Hou J, Wang Y, Shi Y (2016) Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ 23:1128–1139
Kim WJ, Shin HL, Kim BS, Kim HJ, Ryoo HM (2020) RUNX2-modifying enzymes: therapeutic targets for bone diseases. Exp Mol Med 52:1178–1184
Leklem JE (1971) Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am J Clin Nutr 24:659–672
Ma J, Chen P, Wang R (2022) G-protein-coupled receptor 124 promotes osteogenic differentiation of BMSCs through the Wnt/β-catenin pathway. In Vitro Cell Dev Biol Anim 58:529–538
Majláth Z, Török N, Toldi J, Vécsei L (2016) Memantine and kynurenic acid: current neuropharmacological aspects. Curr Neuropharmacol 14:200–209
Michalowska M, Znorko B, Kaminski T, Oksztulska-Kolanek E, Pawlak D (2015) New insights into tryptophan and its metabolites in the regulation of bone metabolism. J Physiol Pharmacol 66:779–791
Nahomi RB, Nam MH, Rankenberg J, Rakete S, Houck JA, Johnson GC, Stankowska DL, Pantcheva MB, MacLean PS, Nagaraj RH (2020) Kynurenic acid protects against ischemia/reperfusion-induced retinal ganglion cell death in mice. Int J Mol Sci 21:1795
Ostapiuk A, Urbanska EM (2022) Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci Ther 28:19–35
Parada-Turska J, Zgrajka W, Majdan M (2013) Kynurenic acid in synovial fluid and serum of patients with rheumatoid arthritis, spondyloarthropathy, and osteoarthritis. J Rheumatol 40:903–909
Perkins MN, Stone TW (1982) An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res 247:184–187
Pierce JL, Begun DL, Westendorf JJ, McGee-Lawrence ME (2019) Defining osteoblast and adipocyte lineages in the bone marrow. Bone 118:2–7
Ramos-Chávez LA, Lugo Huitrón R, González Esquivel D, Pineda B, Ríos C, Silva-Adaya D, Sánchez-Chapul L, Roldán-Roldán G, Pérez de la Cruz V (2018) Relevance of alternative routes of kynurenic acid production in the brain. Oxid Med Cell Longev 2018:5272741
Rejdak R, Junemann A, Grieb P, Thaler S, Schuettauf F, Chorągiewicz T, Zarnowski T, Turski WA, Zrenner E (2011) Kynurenic acid and kynurenine aminotransferases in retinal aging and neurodegeneration. Pharmacol Rep 63:1324–1334
Roth W, Zadeh K, Vekariya R, Ge Y, Mohamadzadeh M (2021) Tryptophan metabolism and gut-brain homeostasis. Int J Mol Sci 22:2973
Schwarcz R, Du F, Schmidt W, Turski WA, Gramsbergen JB, Okuno E, Roberts RC (1992) Kynurenic acid: a potential pathogen in brain disorders. Ann N Y Acad Sci 648:140–153
Solvang SH, Nordrehaug JE, Aarsland D, Lange J, Ueland PM, McCann A, Midttun Ø, Tell GS, Giil LM (2019) Kynurenines, neuropsychiatric symptoms, and cognitive prognosis in patients with mild dementia. Int J Trp Res 12:1178646919877883
Stone TW, Stoy N, Darlington LG (2013) An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci 34:136–143
Tomaszewska E, Muszyński S (2019) Chronic dietary supplementation with kynurenic acid, a neuroactive metabolite of tryptophan, decreased body weight without negative influence on densitometry and mandibular bone biomechanical endurance in young rats. PLoS One 14:e0226205
Tu KN, Lie JD, Wan CKV, Cameron M, Austel AG, Nguyen JK, Van K, Hyun D (2018) Osteoporosis: a review of treatment options. Phar Ther Peer Rev J F Mgt 43:92–104
Tuboly G, Tar L, Bohar Z, Safrany-Fark A, Petrovszki Z, Kekesi G, Vecsei L, Pardutz A, Horvath G (2015) The inimitable kynurenic acid: the roles of different ionotropic receptors in the action of kynurenic acid at a spinal level. Brain Res Bull 112:52–60
Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L (2006) Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem 281:22021–22028
Wei W, Wan Y (2011) Thiazolidinediones on PPARγ: the roles in bone remodeling. PPAR Res 2011:867180
Wirthgen E, Hoeflich A, Rebl A, Günther J (2017) Kynurenic acid: the Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front Immunol 8:1957
Wyant GA, Yu W, Doulamis IP, Nomoto RS, Saeed MY, Duignan T, McCully JD, Kaelin WG Jr (2022) Mitochondrial remodeling and ischemic protection by G protein-coupled receptor 35 agonists. Science (New York, NY) 377:621–629
Yuan Z, Li Q, Luo S, Liu Z, Luo D, Zhang B, Zhang D, Rao P, Xiao J (2016) PPARγ and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther 11:216–225
Zakrocka I, Załuska W (2022) Kynurenine pathway in kidney diseases. Pharmacol Rep 74:27–39
Zarnowski T, Tulidowicz-Bielak M, Zarnowska I, Mitosek-Szewczyk K, Wnorowski A, Jozwiak K, Gasior M, Turski WA (2017) Kynurenic acid and neuroprotective activity of the ketogenic diet in the eye. Curr Med Chem 24:3547–3558
Zhang Y, Shi T, He Y (2021) GPR35 regulates osteogenesis via the Wnt/GSK3β/β-catenin signaling pathway. Biochem Biophys Res Commun 556:171–178
Author information
Authors and Affiliations
Contributions
WR designed the study and supervised the data collection. MJW and CP performed the experiments and DBJ prepared the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All the experimental procedures were approved by the Ethics Committee of The First Hospital of Yulin.
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, J., Chen, P., Deng, B. et al. Kynurenic acid promotes osteogenesis via the Wnt/β-catenin signaling. In Vitro Cell.Dev.Biol.-Animal 59, 356–365 (2023). https://doi.org/10.1007/s11626-023-00774-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-023-00774-2