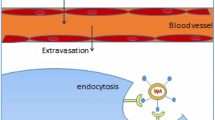
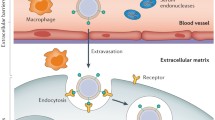
Abstract
Gene transfer and gene therapy studies require high-efficiency gene delivery reagents. By transferring the piece of DNA that we are interested in, we can alter the expression of certain gene or genes to further characterize its role in the cell function or in the organism’s development, metabolism, immune system, etc. Transfection reagents that enable efficient delivery of the DNA to the cells are important tools in the molecular and cellular biology studies. There are chemical products and tools that have been used for transfection of the cells but they are not as efficient as desired or they can induce cytotoxicity. It is crucial to design and generate new transfection reagents to further support the field of biotechnology, molecular studies, cellular biology, and in vitro studies relying on them. The more efficient and the less cytotoxic compounds will be especially useful for the field. We synthesized a new set of benzimidazole-based transfection reagents that have higher efficiency to carry GFP expressing plasmid in to the mammalian cells compared with the commercially available ones with low cytotoxicity. GFP expression levels were tracked by flow cytometry to determine the transfection efficiencies. Benzimidazole-based transfection reagents can be safely used for transfection studies in tissue culture as well as in gene therapy applications due to their high efficiency in the gene transfer to the mammalian cells.




Similar content being viewed by others
References
Ayaz F (2018) Ruthenium pyridyl thiocyanate complex increased the production of pro-inflammatory TNFα and IL1β cytokines by the LPS stimulated mammalian macrophages in vitro. Mol Biol Rep 45:2307–2312
Ayaz F, Ugur N, Ocakoglu K, Ince M (2019) Photo-induced anti-inflammatory activities of chloro substituted subphthalocyanines on the mammalian macrophages in vitro. Photodiagn Photodyn Ther 25:499–503
Brazas RM, Hagstrom JE (2005) Delivery of small interfering RNA to mammalian cells in culture by using cationic lipid/polymer-based transfection reagents. Methods Enzymol 392:112–124
Carter PJ, Samulski RJ (2000) Adeno-associated viral vectors as gene delivery vehicles. Int J Mol Med 6:17–27
Cevik UA, Saglik BN, Ozkay Y (2017) Synthesis of new fluoro-benzimidazole derivatives as an approach towards the discovery of novel intestinal antiseptic drug candidates. Curr Pharm Des 23:2276–2286
Cuerrier CM, Lebel R, Grandbois M (2007) Single cell transfection using plasmid decorated AFM probes. Biochem Biophys Res Commun 355:632–636
Dass CR, Walker TL, Burton MA (2002) Liposomes containing cationic dimethyl dioctadecyl ammonium bromide formulation, quality control and lipofection efficiency. Drug Deliv 9:11–18
Davidse LC (1986) Benzimidazole fungicides: mechanism of action and biological impact. Annu Rev Phytopathol 24:43–65
Díaz-Chiguer DL, Hernández-Luis F, Nogueda-Torres B, Castillo R, Reynoso-Ducoing O, Hernández-Campos A, Ambrosio JR (2014) JVG9, a benzimidazole derivative, alters the surface and cytoskeleton of Trypanosoma cruzi bloodstream trypomastigotes. Mem Inst Oswaldo Cruz 109:757–760
Ehrhardt C, Schmolke M, Matzke A, Knoblauch A, Will C, Wixler V, Ludwing S (2006) Polyethylenimine, a cost-effective transfection reagent. Sign Transduct 6:179–184
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 441:494–468
Elnima EI, Zubair MU, Al-Badr AA (1981) Antibacterial and antifungal activities of benzimidazole and benzoxazole derivatives. Antimicrob Agents Chemother 19:29–32
Gao X, Huang L (1991) A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem Biophys Res Commun 179:280–285
Geisse S, Henke M (2005) Large-scale transient transfection of mammalian cells: A newly emerging attractive option for recombinant protein production. J Struct Funct Genom 6:165–170
Ghosh YK, Visweswariah SS, Bhattacharya S (2000) Nature of linkage between the cationic headgroup and cholesteryl skeleton controls gene transfection efficiency. FEBS Lett 473:341–344
Grunweller A, Hartmann RK (2005) RNA interference as a gene-specific approach for molecular medicine. Curr Med Chem 12:3143–3161
Hamilton AJ, Baulcombe DC (1999) A species of small antisense RNA in post transcriptional gene silencing in plants. Science 286:950–952
Harpe AV, Petersen H, Li Y et al (2000) Characterization of commercially available and synthesized polyethylenimines for gene delivery. J Control Release 69:309–322
Holmen SL, Vanbrocklin MW, Eversole RR, Stapleton SR, Ginsberg LC (1995) Efficient lipid-mediated transfection of DNA into primary rat hepatocytes. In Vitro Cell Dev Biol- Animal 31:347–351
Hunt MA, Currie MJ, Robinson BA, Dachs GU (2010) Optimizing transfection of primary human umbilical vein endothelial cells using commercially available chemical transfection reagents. J Biomol Tech 21:66–72
Karaburun AC, Kaya Cavusoglu KB, Acar U et al (2019) Synthesis and antifungal potential of some novel benzimidazole-1,3,4-oxadiazole compounds. Molecules 24:191–205
Katiyar SK, Gordon VR, McLaughlin GL, Edlind TD (1994) Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrob Agents Chemother 38:2086–2090
Khabnadideh S, Rezaei Z, Pakshir K, Zomorodian K, Ghafari N (2012) Synthesis and antifungal activity of benzimidazole, benzotriazole and aminothiazole derivatives. Res Pharm Sci 7:65–72
Kiefer K, Clement J, Garidel P, Peschka-Suss R (2004) Transfection efficiency and cytotoxicity of nonviral gene transfer reagents in human smooth muscle and endothelial cells. Pharm Res 21:1009–1017
Kim TK, Eberwine JH (2010) Mammalian cell transfection: the present and the future. Anal Bioanal Chem 397:3173–3178
Kircheis R, Wightman L, Wagner E (2001) Design and gene delivery activity of modified polyethylenimines. Adv Drug Deliv Rev 31:348
Kirschner M, Monrose V, Paluch M, Techodamrongsin N, Rethwilm A, Moore JP (2006) Techodamrongsin N,The production of cleaved, trimeric human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein vaccine antigens and infectious pseudoviruses using linear polyethylenimine as a transfection reagent. Protein Expr Purif 48:61–68
Lacey E (1990) Mode of action of benzimidazoles. Parasitol Today 6:112–115
Liu Y, Fan Z, Li K, Deng F, Xiong Y, Liang M, Ge J (2017) An optimized gene transfection system in WERI-Rb1 cells. Int J Mol Med 40:801–813
Martinou I, Fernandez PA, Missotten M, White E, Allet B, Sadoul R, Martinou JC (1995) Viralproteins E1B19K and p35 protect sympathetic neurons from cell death induced by NGF deprivation. J Cell Biol 128:201–208
McBain SC, Yiu HHP, El Haj A (2007) Polyethyleneimine functionalized iron oxide nanoparticles as agents for DNA delivery and transfection. J Mater Chem 7:2561–2565
McGregor C, Perrin C, Monck M, Camilleri P, Kirby AJ (2001) Rational Approaches to the Design of Cationic Gemini Surfactants for Gene Delivery. J Am Chem Soc 123:6215–6220
Melissa MF, Hoversten KE, Powers JM, Trobridge GD, Rodgers BD (2013) Genetic manipulation of myoblasts and a novel primary myosatellite cell culture system: comparing and optimizing approaches. FEBS J 280:827–840
Mentese E, Doğan IS, Kahveci B (2013) Green protocol: solvent- and catalyst-free synthesis of benzimidazole derivatives via microwave technique. Chem Heterocycl Compd 49:1136–1140
Mobinikhaledi A, Hamta A, Kalhor M, Shariatzadeh M (2014) Simple synthesis and biological evaluation of some benzimidazoles using sodium hexafluroaluminate, Na3AlF6, as an efficient catalyst. Iran J Pharm Res 13:95–101
Moyano DF, Liu Y, Ayaz F, Hou S, Duncan B, Osborne BA, Rotello VM (2016) Immunomodulatory effects of coated gold nanoparticles in LPS-stimulated in vitro and in vivo murine model systems. Chem 1:320–327
Nannapaneni D, Gupta AV, Nannapaneni DT, Reddy M (2010) Synthesis, characterization, and biological evaluation of benzimidazole derivatives as potential anxiolytics. J Young Pharm 2:273–279
Nimesh S, Halappanavar S, Kaushik NK, Kumar P (2015) Advances in gene delivery systems. Biomed Res Int 2015:610342–610344
Ouahrouch A, Ighachane H, Taourirte M, Engels JW, Sedra MH, Lazrek HB (2014) Benzimidazole-1,2,3-triazole hybrid molecules: synthesis and evaluation for antibacterial/antifungal activity. Arch Pharm 347:748–755
Pampinella F, Lechardeur D, Zanetti E, MacLachlan I, Benharouga M, Lukacs GL, Vitiello L (2002) Analysis of differential lipofection efficiency in primary and established myoblasts. Mol Ther 5:161–169
Parelkar SS, Chan-Seng D, Emrick T (2011) Reconfiguring polylysine architectures for controlling polyplex binding and non-viral transfection. Biomaterial 32:2432–2444
Park H, Yang F, Cho S (2012) Nonviral delivery of genetic medicine for therapeutic angiogenesis. Adv Drug Deliv Rev 64:40–52
Pautz GE, Yang ZY, Wu BY, Gao X, Huang L, Nabel GJ (1993) Immunitherapy of malignancy by in vivo gene transfer into tumors. Proc Natl Acad Sci 90:4645–4649
Recillas-Targa F (2006) Multiple strategies for gene transfer, expression, knockdown, and chromatin influence in mammalian cell lines and transgenic animals. Mol Biotechnol 34:337–354
Santos AD, Kaïm LE, Grimaud L (2013) Metal-free aerobic oxidation of benzazole derivatives. Org Biomol Chem 11:3282–3287
Seow Y, Wood MJ (2009) Biological gene delivery vehicles: beyond viral vectors. Mol Ther 17:767–777
Shin JM, Sachs G, Cho YM, Garst M (2009) 1-Arylsulfonyl-2-(pyridylmethylsulfinyl) benzimidazoles as new proton pump inhibitor prodrugs. Molecules 14:5247–5280
Simberg D, Hirsch-Lerner D, Nissim R, Barenholz Y (2000) Comparison of different commercially available cationic lipid-based transfection kits. J Liposome Res 10:1–13
Slowing II, Vivero-Escoto JL, Trewyn BG, Lin VSY (2010) Mesoporous silica nanoparticles: structural design and applications. J Mater Chem 20:7924–7937
Vacik J, Dean BS, Zimmer WE, Dean DA (1999) Cell-specific nuclear import of plasmid DNA. Gene Ther 6:1006–1014
Wang Y, Sarris K, Sauer DR, Djuric SW (2006) A simple and efficient one step synthesis of benzoxazoles and benzimidazoles from carboxylic acids. Tetrahedron Lett 47:4823–4826
Yamamoto A, Kormann M, Rosenecker J, Rudolph C (2009) Current prospects form RNA gene delivery. Eur J Pharm Biopharm 71:484–489
Funding
This study was financially supported by 2018-2-AP4-2919 BAP Project of Mersin University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors Furkan Ayaz and Oztekin Algul filed a patent application to the Turkish Patent Institute. Other than the patent application, the authors have no conflict of interest to declare.
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Ayaz, F., Ersan, R.H., Kuzu, B. et al. New-Generation Benzimidazole-Based Plasmid Delivery Reagents with High Transfection Efficiencies on the Mammalian Cells. In Vitro Cell.Dev.Biol.-Animal 56, 34–41 (2020). https://doi.org/10.1007/s11626-019-00418-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-019-00418-4