Abstract
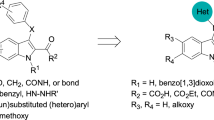
Anticancer role of oxindole compounds is well documented. Here, we synthesized new derivatives of 3-hydroxy-2-oxindole functionalized at position 3 (1a–f) which are expected to have antiproliferative activity in cancer cells. Human prostate cancer cell line (DU145) was treated with the synthesized derivatives at 40-μM concentration for 24, 48, and 72 h. Compounds 1-ethyl-3-hydroxy-1,1′,3,3′-tetrahydro-2H,2′H-3,3′-biindole-2,2′-dione (1d), 5-bromo-1-ethyl-3-hydroxy-1,1′,3,3′-2H,2′H-3,3′-biindole-2,2′-dione (1e), and 5-chloro-1-ethyl-3-hydroxy-1,1′,3,3′-tetrahydro-2H,2′H-3,3′-biindole-2,2′-dione (1f) were found to significantly reduce DU145 cell viability at 48 and 72 h whereas no significant changes were observed up to 24 h. The compounds 1e and 1f showed the most cytotoxicity effect and had a similar antiproliferative activity on DU145 cell line. They have halogen and ethyl substitutions at positions 5 and 1, respectively. The IC50 of compound 1e for DU145 and A375 cells at 48 h was determined. The apoptotic effects and cell cycle progression of compound 1e at 1/2 × IC50 (55 μM) concentration in DU145 cells were investigated by nuclei staining, comet assay, flow cytometry, and scanning electron microscopy (SEM). The results obtained showed that this compound increased the percentage of tail DNA, increased the occurrence of the sub-G1 phase, and induced G2M arrest and apoptosis in DU145 cells after exposure for 48 h to a 55-μM concentration. The SEM images revealed cell contraction at 24 h, cell condensation, plasma membrane blebbing, and formation of apoptotic bodies at 48 and 72 h. These observations suggest that the antiproliferative activity of compound 1e may be to induce apoptosis in DU145 cells.








Similar content being viewed by others
References
Aikawa K, Mimura S, Numata Y, Mikami K (2011) Palladium-catalyzed enantioselective ene and aldol reactions with isatins, keto esters, and diketones: reliable approach to chiral tertiary alcohols. Eur J Org Chem 1:62–65
Bacher N, Tiefenthaler M, Sturm S, Stuppner H, Ausserlechner MJ, Kofler R, Konwalinka G (2006) Oxindole alkaloids from Uncaria tomentosa induce apoptosis in proliferating, G0/G1 arrested and bcl-2-expressing acute lymphoblastic leukaemia cells. Brit J Haematol 132:615–622
Bagnall P (2014) Diagnosis and treatment of prostate cancer. Nurs Times 110:12–15
Dey SK, Bose D, Hazra A, Naskar S, Nandy A, Munda RN, Das S, Chatterjee N, Mondal NB, Banerjee S, Saha KD (2013) Cytotoxic activity and apoptosis-inducing potential of di-spiropyrrolidino and di-spiropyrrolizidino oxindole andrographolide derivatives. PLoS One 8:e58055. https://doi.org/10.1371/journal.pone.0058055
Djavan B, Nasu Y (2001) Prostate cancer gene therapy—what have we learned and where are we going? Rev Urol 3:179–186
Gangar M, Kashyap N, Kumar K, Goyal S, Nair VA (2015) Imidazolidinone based chiral auxiliary mediated acetate aldol reactions of isatin derivatives and stereoselective synthesis of 3-substituted-3-hydroxy-2-oxindoles. Tetrahedron Lett 56:7074–7081
Gerebtzoff G, Li-Blatter X, Fischer H, Frentzel A, Seelig A (2004) Halogenation of drugs enhances membrane binding and permeation. Chembiochem 5:676–684
Hernandes MZ, Cavalcanti SM, Moreira DR, de Azevedo Junior WF, Leite AC (2010) Halogen atoms in the modern medicinal chemistry: hints for the drug design. Curr Drug Targets 11:303–314
Jewett MA, Fleshner N, Klotz LH, Nam RK, Trachtenberg J (2003) Radical prostatectomy as treatment for prostate cancer. Can Med Assoc J 168:44–45
Jha M, Shelke GM, Kumar A (2014) Catalyst-free one-pot tandem reduction of oxo and Ene/Yne functionalities by hydrazine: synthesis of substituted oxindoles from isatins. Eur J Org Chem 16:3334–3336
Kamal A, Ramakrishna G, Raju P, Rao AS, Viswanath A, Nayak VL, Ramakrishna S (2011) Synthesis and anticancer activity of oxindole derived imidazo [1, 5-a] pyrazines. Eur J Med Chem 46:2427–2435
Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA (2013) Mutational landscape and significance across 12 major cancer types. Nature 502:333–339
Leoni A, Locatelli A, Morigi R, Rambaldi M, Cappadone C, Farruggia G, Iotti S, Merolle L, Zini M, Stefanelli C (2014) Substituted E-3-(3-indolylmethylene) 1, 3-dihydroindol-2-ones with antiproliferative activity. Study of the effects on HL-60 leukemia cells. Eur J Med Chem 79:382–390
Lowe SW, Ruley HE, Jacks T, Housman DE (1993) p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74:957–967
Lüpertz R, Wätjen W, Kahl R, Chovolou Y (2010) Dose- and time-dependent effects of doxorubicin on cytotoxicity, cell cycle and apoptotic cell death in human colon cancer cells. Toxicology 271:115–121
Ma Z, Hou L, Jiang Y, Chen Y, Song J (2014) The endogenous oxindole isatin induces apoptosis of MCF-7 breast cancer cells through a mitochondrial pathway. Oncol Rep 32:2111–2117
Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E (2012) European cancer mortality predictions for the year 2012. Ann Oncol 23:1044–1052
Mohammadi S, Khoei S, Mahdavi SR (2012) The combination effect of poly (lactic-co-glycolic acid) coated iron oxide nanoparticles as 5-fluorouracil carrier and X-ray on the level of DNA damages in the DU 145 human prostate carcinoma cell line. J Bionanosci 6:23–27
Ogura Y, Akakura M, Sakakura A, Ishihara K (2013) Enantioselective cyanoethoxycarbonylation of isatins promoted by a Lewis base–Brønsted acid cooperative catalyst. Angew Chem Int Ed 52:8299–8303
Peddibhotla S (2009) 3-Substituted-3-hydroxy-2-oxindole, an emerging new scaffold for drug discovery with potential anti-cancer and other biological activities. Curr Bioact Compd 5:20–38
Pérès EA, Gérault AN, Valable S, Roussel S, Toutain J, Divoux D, Guillamo JS, Sanson M, Bernaudin M, Petit E (2015) Silencing erythropoietin receptor on glioma cells reinforces efficacy of temozolomide and X-rays through senescence and mitotic catastrophe. Oncotarget 6:2101–2119
Ribeiro CJ, Amaral JD, Rodrigues CM, Moreira R, Santos MM (2014) Synthesis and evaluation of spiroisoxazoline oxindoles as anticancer agents. Bioorgan Med Chem 22:577–584
Senadi GC, Hu WP, Boominathan SSK, Wang JJ (2015) Palladium(0)-catalyzed single and double isonitrile insertion: a facile synthesis of benzofurans, indoles, and isatins. Chem Eur J 21:998–1003
Siegal G, Eiso AB, Schultz J (2007) Integration of fragment screening and library design. Drug Discov Today 12:1032–1039
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA-Cancer J Clin 64:9–29
Suzen S, Buyukbingol E (2000) Anti-cancer activity studies of indolalthiohydantoin (PIT) on certain cancer cell lines. II Farmaco 55:246–248
Thakur PB, Meshram HM (2014) “On water” catalyst-free, column chromatography-free and atom economical protocol for highly diastereoselective synthesis of novel class of 3-substituted, 3-hydroxy-2-oxindole scaffolds at room temperature. RSC Adv 4:5343–5350
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Tice RR, Strauss GH (1995) The single cell gel electrophoresis/comet assay: a potential tool for detecting radiation-induced DNA damage in humans. Stem Cells 13:207–214
Funding
This project was supported by the Ferdowsi University of Mashhad, grant number 3/32602.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Nazemi Moghaddam, M., Jalal, R. & Zeraatkar, Z. Synthesis and antiproliferative and apoptosis-inducing activity of novel 3-substituted-3-hydroxy-2-oxindole compounds. In Vitro Cell.Dev.Biol.-Animal 54, 61–70 (2018). https://doi.org/10.1007/s11626-017-0204-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-017-0204-8