Abstract
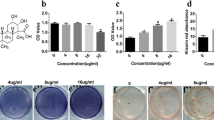
Total Panax notoginseng saponin (PNS) has been extensively used to treat a variety of diseases, such as bone fractures, soft tissue injuries, etc. In this study, mouse calvaria-original osteoblastic MC3T3-E1 cells were cultured in various concentrations of PNS (0.005–5 mg/mL) during the period (1, 5, 14, and 23 d). At the endpoint, the osteogenic capacity of MC3T3-E1 cells was investigated by measuring the alkaline phosphatase (ALP) activity, the deposited calcium, and the expression of osteogenic-related markers, including bone collagen type 1 (Col1) and osteocalcin (OCN). Compared with all groups in each period, the most pronounced effect was observed at the concentration range between 0.05 and 0.5 mg/mL (P < 0.05) and the cell proliferation with PNS treatment was found during the whole osteogenic period. Moreover, cellular ALP activity with PNS was increased during 7, 14, and 21 d and cell mineralization with PNS was enhanced in 14 and 21 d. Furthermore, the differentiation markers Col1 and OCN increased in the PNS-treated cells. Our work suggests that PNS may stimulate the osteogenesis process which contains osteoblastic proliferation, differentiation, and mineralization by increasing cellular ALP activity, extracellular matrix mineralization, and osteoblast-associated molecules in the osteoblasts.






Similar content being viewed by others
References
Aubin JE (1998) Bone stem cells. J Cell Biochem Suppl 30–31:73–82
Bahlous A, Kalai E, Hadj Salah M, Bouzid K, Zerelli L (2006) Biochemical markers of bone remodeling: recent data of their applications in managing postmenopausal osteoporosis. Tunis Med 84:751–757
Baron R, Rawadi G (2007) Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148:2635–2643
Bord S, Frith E, Ireland DC, Scott MA, Craig JI, Compston JE (2005) Megakaryocytes modulate osteoblast synthesis of type-l collagen, osteoprotegerin, and RANKL. Bone 36:812–819
Bruderer M, Richards RG, Alini M, Stoddart MJ (2014) Role and regulation of RUNX2 in osteogenesis. Eur Cell Mater 28:269–286
Cho YE, Alcantara E, Kumaran S, Son KH, Sohn HY, Lee JH, Choi CS, Ha TY, Kwun IS (2010) Red yeast rice stimulates osteoblast proliferation and increases alkaline phosphatase activity in MC3T3-E1 cells. Nutr Res 30:501–510
Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G (1996) Increased bone formation in osteocalcin-deficient mice. Nature 382:448–452
Duquet N (2014) Osteoporosis: treatment and pharmaceutical care. J Pharm Belg 2:14–24
Fan TP, Yeh JC, Leung KW, Yue PY, Wong RN (2006) Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci 27:297–309
Franceschi RT, Ge C, Xiao G, Roca H, Jiang D (2009) Transcriptional regulation of osteoblasts. Cells Tissues Organs 189:144–152
Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, Roman-Roman S (2001) Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone 28:491–498
Gao YT, Zhang WB, Yang LR, Wang XM, Yang YL (2008) Effects of Panax notoginseng saponins on antioxidation and preventing DNA damage caused by hydroxyl radical. Zhong Yao Cai 31:1399–1402
Hong SJ, Wan JB, Zhang Y, Hu G, Lin HC, Seto SW, Kwan YW, Lin ZX, Wang YT, Lee SM (2009) Angiogenic effect of saponin extract from Panax notoginseng on HUVECs in vitro and zebrafish in vivo. Phytother Res 23:677–686
Kozawa O, Hatakeyama D, Uematsu T (2002) Divergent regulation by p44/p42 MAP kinase and p38 MAP kinase of bone morphogenetic protein-4-stimulated osteocalcin synthesis in osteoblasts. J Cell Biochem 84:583–589
Kwun IS, Cho YE, Lomeda RA, Shin HI, Choi JY, Kang YH, Beattie JH (2010) Zinc deficiency suppresses matrix mineralization and retards osteogenesis transiently with catch-up possibly through Runx 2 modulation. Bone 46:732–741
Leboime A, Confavreux CB, Mehsen N, Paccou J, David C, Roux C (2010) Osteoporosis and mortality. Joint, Bone, Spine 77(Suppl 2):S107–112
Li L, Sheng Y, Zhang J, Guo D (2005a) Determination of four active saponins of Panax notoginseng in rat feces by high-performance liquid chromatography. J Chromatogr Sci 43:421–425
Li L, Zhang JL, Sheng YX, Guo DA, Wang Q, Guo HZ (2005b) Simultaneous quantification of six major active saponins of Panax notoginseng by high-performance liquid chromatography-UV method. J Pharm Biomed Anal 38:45–51
Li XD, Wang JS, Chang B, Chen B, Guo C, Hou GQ, Huang DY, Du SX (2011) Panax notoginseng saponins promotes proliferation and osteogenic differentiation of rat bone marrow stromal cells. J Ethnopharmacol 134:268–274
Lind T, Sundqvist A, Hu L, Pejler G, Andersson G, Jacobson A, Melhus H (2013) Vitamin a is a negative regulator of osteoblast mineralization. PLoS One 8:e82388
Liu Y, Zhang HG, Jia Y, Li XH (2010) Panax notoginseng saponins attenuate atherogenesis accelerated by zymosan in rabbits. Biol Pharm Bull 33:1324–1330
Martin TJ, Sims NA, Ng KW (2008) Regulatory pathways revealing new approaches to the development of anabolic drugs for osteoporosis. Osteoporos Int 19:1125–1138
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P (2002) The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem 277:5330–5338
Ozeki K, Aoki H, Fukui Y (2008) The effect of adsorbed vitamin D and K to hydroxyapatite on ALP activity of MC3T3-E1 cell. J Mater Sci Mater Med 19:1753–1757
Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT (2006) BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res 21:637–646
Putnam SE, Scutt AM, Bicknell K, Priestley CM, Williamson EM (2007) Natural products as alternative treatments for metabolic bone disorders and for maintenance of bone health. Phytother Res 21:99–112
Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ (1992) Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res 7:683–692
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287
Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, Spelsberg TC (2000) Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res 60:6001–6007
Rhule A, Navarro S, Smith JR, Shepherd DM (2006) Panax notoginseng attenuates LPS-induced pro-inflammatory mediators in RAW264.7 cells. J Ethnopharmacol 106:121–128
Sharan K, Mishra JS, Swarnkar G, Siddiqui JA, Khan K, Kumari R, Rawat P, Maurya R, Sanyal S, Chattopadhyay N (2011) A novel quercetin analogue from a medicinal plant promotes peak bone mass achievement and bone healing after injury and exerts an anabolic effect on osteoporotic bone: the role of aryl hydrocarbon receptor as a mediator of osteogenic action. J Bone Miner Res 26:2096–2111
Shen Y, Li YQ, Li SP, Ma L, Ding LJ, Ji H (2010) Alleviation of ovariectomy-induced osteoporosis in rats by Panax notoginseng saponins. J Nat Med 64:336–345
Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT (1999) Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res 14:893–903
Wang P, Cui J, Du X, Yang Q, Jia C, Xiong M, Yu X, Li L, Wang W, Chen Y, Zhang T (2014) Panax notoginseng saponins (PNS) inhibits breast cancer metastasis. J Ethnopharmacol 154:663–671
Wang W, Olson D, Cheng B, Guo X, Wang K (2012) Sanguis draconis resin stimulates osteoblast alkaline phosphatase activity and mineralization in MC3T3-E1 cells. J Ethnopharmacol 142:168–174
Yamamoto N, Akiyama S, Katagiri T, Namiki M, Kurokawa T, Suda T (1997) Smad1 and smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts. Biochem Biophys Res Commun 238:574–580
Yin LM, Wang X, Qian XD, Lin XJ, Chen XH, Gao RL (2012) Effects of Panax notoginseng saponins on proliferation and differentiation in NIH3T3 cells. Chin J Integr Med 18:616–620
Zheng H, Liu C, Ou Y, Zhang Y, Fu X (2013) Total saponins of Panax notoginseng enhance VEGF and relative receptors signals and promote angiogenesis derived from rat bone marrow mesenchymal stem cells. J Ethnopharmacol 147:595–602
Acknowledgments
This study was supported by the Natural Science Foundation of China (No. 81001225), the international Co-operative Fund in Xian Jiaotong University (No. 08143004), the Fundamental Research Funds for the Central Universities (No. 08140003), and Integrative Medicine Research and Innovation Team of Degenerative Bone Disease Prevention, Shaanxi Traditional Chinese Medicine College (2013KCT-26).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Zhe Ji and Yizhao Cheng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ji, Z., Cheng, Y., Yuan, P. et al. Panax notoginseng stimulates alkaline phosphatase activity, collagen synthesis, and mineralization in osteoblastic MC3T3-E1 cells. In Vitro Cell.Dev.Biol.-Animal 51, 950–957 (2015). https://doi.org/10.1007/s11626-015-9915-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-015-9915-x