Abstract
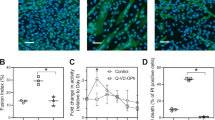
The concentration-dependent effect of spermine was investigated on the spermine-induced generation of multilayer myotube sheets (MMTS) from mouse embryoid bodies (EBs). During spermine treatment for 24 h, a monolayer cell sheet that had already grown radially from the periphery of an EB was exfoliated. The exfoliation was inhibited by z-VAD.fmk, indicating the occurrence of apoptosis, and inhibited also by aminoguanidine, indicating the involvement of amine oxidase. Following the exfoliation, the cell growth restarted from the fresh periphery of EB in a spermine-free medium and finally formed MMTS. To analyze the contribution of apoptosis to the cell death causing exfoliation, the numbers of apoptotic, necrotic, and 2nd apoptotic cells were counted by staining with Annexin V-Cyanine-3 (AVC3) and 7-aminoactinomycin (7AAC). AVC3-positive, 7AAC-positive, and AVC3/7AAC doubly positive cells were assigned as apoptotic, necrotic, and 2nd necrotic cells, respectively. The relative number of apoptotic and 2nd necrotic cells (N A + N A/7) to the total number of dying cells (N T) was 84 ∼ 94%, which was independent of spermine concentration in the range from 0.1 to 2.0 mM. The MMTS generation rate at the final stage, however, was dependent on the spermine concentration. It was 60 ∼ 80% in the range from 0.1 to 1.5 mM, while it decreased sharply to 1% at 2 mM. This suggests another role of spermine in the MMTS generation in addition to the induction of apoptosis. This 2nd role seems to be inhibited at a spermine concentration higher than a critical limit between 1.5 and 2.0 mM.






Similar content being viewed by others
References
Atamaniuk J, Ruzicka K, Stuhlmeier KM, Karimi A, Eigner M, Mueller MM (2006) Cell-free plasma DNA: a marker for apoptosis during hemodialysis. Clin Chem 52:523–526
Degterev A, Yuan J (2008) Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol 9:378–390
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43–50
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148:2207–2216
Golstein P, Kroemer G (2007) Cell death by necrosis: towards a molecular definition. Trends Biochem Sci 32:37–43
Grancara S, Martinis P, Manente S, García-Argáez AN, Tempera G, Bragadin M, Via LD, Agostinelli E, Toninello A (2014) Bidirectional fluxes of spermine across the mitochondrial membrane. Amino Acids 46:671–679
Inoue S, Browne G, Melino G, Cohen GM (2009) Ordering of caspase in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ 16:1053–1061
Kajstura M, Halicka HD, Pryjma J, Darzynkiewicz Z (2007) Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete “sub-G1” peaks on DNA content histograms. Cytom Part A 71:125–131
Kunimoto S, Miura K, Iinuma H, Takeuchi T, Umezawa H (1985) Cytotoxicity of spergualin and amine oxidase activity in medium. J Antibiot 38:899–903
Kurokawa M, Kornbluth S (2009) Caspases and kinases in a death grip. Cell 138:838–854
Lee SH, Meng XW, Flatten KS, Loegering DA, Kaufmann SH (2013) Phosphatidylserine exposure during apoptosis reflects bidirectional trafficking between plasma membrane and cytoplasm. Cell Death Differ 20:64–76
Nagata S (2000) Apoptotic DNA fragmentation. Exp Cell Res 256:12–18
Niu G, Chen X (2010) Apoptosis imaging: beyond annexin V. J Nucl Med 51:1659–1662
Salvesen GS, Riedl SJ (2008) Caspase mechanisms. Adv Exp Med Biol 615:13–23
Sasaki T, Matsuoka H, Saito M (2008a) Generation of a multi-layer muscle fiber sheet from mouse ES cells by the spermine action at specific timing and concentration. Differentiation 76:1023–1030
Sasaki T, Oh K-B, Matsuoka H, Saito M (2008b) Effect of Panax ginseng components on the differentiation of mouse embryonic stem cells into cardiac-like cells. Yakugaku Zasshi-J Pharm Soc Jpn 128:461–467 (in Japanese)
Schiller M, Blank N, Heyder P, Herrmann M, Gaipl US, Kalden JR, Lorenz HM (2005) Induction of apoptosis by spermine-metabolites in primary human blood cells and various tumor cell lines. Apoptosis 10:1151–1162
Sebastian TT, Baldridge RD, Xu P, Graham TR (2012) Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim Biophys Acta Mol Cell Biol Lipids 1821:1068–1077
Sharmin S, Sakata K, Kashiwagi K, Ueda S, Iwasaki S, Shirahata A, Igarashi K (2001) Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem Biophys Res Commun 282:228–235
Slee EA, Zhu H, Chow SC, MacFarlane M, Nicholson DW, Cohen GM (1996) Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.fmk) inhibits apoptosis by blocking the processing of CPP32. Biochem J 315:21–24
van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytom Part A 31:1–9
Zhang L, Gao X, Yuan X, Dong H, Zhang Z, Wang S (2014) Mitochondrial calcium uniporter opener spermine attenuates the cerebral protection of diazoxide through apoptosis in rats. J Stroke Cerebrovasc Dis 23:829–835
Acknowledgments
This work was partially funded by a grant to H. Matsuoka from CREST of Japan Science and Technology Agency on the research subject The High Throughput Creation of Disease Model Cells and the Analysis of Their Function. The work was also supported in part by the Strategic Research Promotion Program, the Ministry of Education, Culture, Sports, Science, and Technology, on the research subject “Development of Next Generation Bioresources.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Natsuki Abe and Ayano Ishida equally contributed to this paper.
Rights and permissions
About this article
Cite this article
Saito, M., Abe, N., Ishida, A. et al. Concentration-dependent effects of spermine on apoptosis and consequent generation of multilayer myotube sheets from mouse embryoid bodies in vitro. In Vitro Cell.Dev.Biol.-Animal 50, 973–981 (2014). https://doi.org/10.1007/s11626-014-9799-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-014-9799-1